Advances in Animal and Veterinary Sciences
Research Article
Comparative Study for Detection of Subclinical Endometritis in Local Cows
R. B. Bedewy, M. A. Rahawy*
Department of Surgery and Theriogenology, College of Veterinary Medicine, University of Mosul, Iraq.
Abstract | The objective of the present study was to determine using the Dramincki estrus detector and to evaluate its efficiency in diagnosis of subclinical endometritis in cows. In addition, the diagnosis of subclinical endometritis with different methods such as assess the efficacy of endometrial cytology to determine the use of percentage of poly morpho-nuclear cells PMNs in cervical mucus, measurement of the PH, White side test and sperm penetration test of the cervical mucus. The study was conducted on 42 cows suffering from regularly repeated estrus located in different regions in Nineveh province from the period of 01/10/2017 to 01/10/2018. All cows were subjected to general clinical examination, cervical and vaginal examination by vaginal speculum, and the Dramincki detector was used to ensure that the cows are in estrum. Samples of cervical mucus were collected from all cows for estimation the percentage of PMNs (12≥18). Whiteside test was utilized to determine changes in cervical mucus color from normal to a light yellowish color. Furthermore, microscopic sperm penetration test was used to determine the ability of sperm to penetrate the cervical mucus by adding a drop of liquid semen close to the mucus sample. Results showed that subclinical endometritis was diagnosed in 16.66% (7/42) of the repeated breeding cows. However, there was no significantly correlation between the age of the cows and both number of estrus, and values of the estrus detector in cows affected with subclinical endometritis. Conversely, there was a significant correlation in the detected subclinical endometritis by endometrial cytology that indicated as an increasing percentage of PMNs up to 12%≥ 18% with the increased age of the affected cows at (5.43± 0.841). Similarly, subclinical endometritis was highly significant associated with the increased PH value of cervical mucus (7.800 ± 0.0577) as compared with unaffected cows (7.271 ± 0.034), change the mucus color to light yellowish, as well as with the mucus penetration ability of sperms, as the majority of sperms died after a short distance of mucus penetration from cows suffered from subclinical endometritis. To some briefly, diagnosis of subclinical endometritis, using pH value, cytological investigation, white side test and sperm penetration test provided a considerable value in field conditions.
Keywords | Subclinical endometritis, pH, Cytology, Whiteside, Sperm penetration
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 17, 2018; Accepted | November 12, 2018; Published | January 30, 2019
*Correspondence | M A Rahawy, Department of Surgery and Theriogenology, College of Veterinary Medicine, University of Mosul, Iraq; Email: mohammedrahawy@yahoo.com
Citation | Bedewy RB, Rahaway MA (2019). Comparative study for detection of subclinical endometritis in local cows. Adv. Anim. Vet. Sci. 7(4): 289-294.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.4.289.294
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Bedewy and Rahawy. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Subclinical endometritis considers as one of the important etiological factors of repeat breeding in cows. Early diagnosis is essential to minimize the economic loss. Diagnosis of infectious repeat breeding cows and isolation of the non-infectious repeat breeding problem is first step to challenge for the field veterinarian. Careful checking of uterine ultrasonography scanning of the uterus, vaginoscopy of vagina, endometrial cytology, uterine mucus and uterine biopsy have been testified as diagnostic approaches to identify endometritis (Barlund et al., 2008). Posing considerable economic loss to dairy farmers. Subclinical endometritis has been the one of major cause the repeat breeding (Arthur et al., 1989).
Repeat breeders are animal cycling normally without any clinical abnormalities, but fail to conceive even after at least three successive inseminations. They have clinically normal reproductive tract, estrous cycles and estrous periods (Arthur et al, 2001).
Subclinical endometritis alters the physico-chemical properties of cervical mucus and: therefore, examination received of cervical mucus for appearance PH. It maybe valuable in its diagnosis (Vijayajan et al., 2007; Hussain et al., 1991).
Cytological examination of reproductive tract is often used to evaluate possible reproductive lesions by cervical cytological examination in cow (Mateus et al., 2002b, Leblanc et al., 2002a) The endometrial and inflammatory cells detected technique were accepted and controlled by cyto-brush technique and mucus smear (Kamanickam et al., 2005b) techniques to evaluate endometrial cytology specially as an aid in the detection of endometritis prepared smear most contain epithelial cells. A ratio of polymorph nuclear cells (PMNs) to epithelial should be calculated. If the ratio is more than 12% PMNs to epithelial cells, subclinical endometritis significantly (Mateus et al., 2002b: Leblanc et al., 2002a).
This clearly indicated the efficacy of white side test WST for detection of subclinical endometritis by boiling point and after cooling the intensity of color changes were graded as normal (no color) mild infection or subclinical endometritis (light yellow color) moderate infection or clinical endometritis (yellow color) severe infection or chronic endometritis (dark yellow color) (Kumar et al., 2015), The color change observed in white side test (WST) in control cows might be due to neutrophil infiltration and metrorrhagia, which are usually seen during estrogen dominance (Ohtani et al., 1993; Raja et al., 2012).
Cervical mucus pH and bacterial load in CM are absolutely correlated with WST and the test is apposite in detection of endometritis at both clinical and subclinical degree. Moreover, White side test can decrease the cost for analyzing endometritis. Detection of endometritis with white side test has clinical importance, and the test can be directed at the field level, when they fail to conceive repeatedly (Rajaet al 2012).
IgA–antisperm antibody (ASA) is highly detectable in cervical mucus (CM) (Wang et al., 2009). ASA in cervical mucus has a great impact on the ability of sperms to penetrate cervical mucus in human (Eggert-krust et al., 1991).There are only a little amount of researches in veterinary field under the influence of ASA in CMon sperm penetration and its impact on fertility rates.The principle aims of the present study are to determine the incidence of subclinical endometritis in local cows using various methods for diagnosis. These methods included the using of dramincki estrus detector, endometrial cytology to determine the percentage of PMNs, measurement the pH, White slide test and sperm penetration testin cervical mucus.
Materials and MethodS
All repeats breeding cows were observed and data (age cows, number of repeated estrus) was recorded. Forty two regular cyclic localcows were presented to the Teaching Veterinary Clinical Service in College of Veterinary Medicine-Mosul University and Ninavah Veterinary hospital; the study was conducted during the one -year period from October 2017 to October 2018. Gynecological examination of these cows revealed no palpable abnormalities of the reproductive organ.
All cows were subjected to detailed general clinical examination, cervical and vaginal examination by vaginal speculum, and the Dramincki detector was used to ensure that the cows are in estrum. With aseptic precautions, using the sterile AI sheath connected with 20ml syringe, cervical mucus, which represented the endometrial secretion, was collected.
All Mucus samples were estimated of PH value by portable PH meter. Endometrial cytology samples were collected from each cow included in this study aspirated from cervical lumen using sterile catheter and transferred into sterile tubes. Then transported to the laboratory at 4c0. Smears were prepared from the cervical mucus onclean glass microscope slide and fixed with absolute methyl alcohol and stained by Right-Giemsastain (Mateus et al., 2002). Endometrial cytological examination of polymorph nuclear cell (PMNs) was evaluated using light microscope at magnifications 100X and 400X.Whiteside test was used to determine changes in cervical mucus coloration from normal to a light yellowish color. One ml of the cervical mucus was mixed with one ml of 5 per cent sodium hydroxide solution in a test tube and heated up to the boiling point and subsequently cooled in running tap water. The appearance of yellow color was considered as the positive indicator of presence the infection. Depending on the intensity of color development, the degree of endometritis was classified as No color (absence of infection), mild yellowcolor change (mild infection or subclinical), intense yellow color (severe infection or clinical) (Anilkumar and Devanathan, 1996). Sperm penetration test was used to determine the ability of sperm to penetrate the cervical mucus by adding a drop from straw contain 20×106 sperm of liquid semen close to the small amount of mucus sample put on the worm slide at 37° C. and showed the sperm penetrated by microscope with dark field at 100X.
Results
Laboratorial examinations revealed that subclinical endometritis was diagnosed in 7/42 (16. 66%) of the repeated breeding local cows. Subclinical endometritis was not diagnosed in cows ranged between 3-8 years old (means 5.00± 0.272). Conversely, subclinical endometritis was diagnosed in cows ranged between 3-9 years (means 5.43± 0.841). Inseminated unaffected cows were returned to estrus for 2-4 times (means 2.80 ± 0.141) due to conception failure. However, return to estrus, after insemination, was detected 2-5 times (means 3.43 ± 0.369) in the affected cows. In unaffected cows, the estimated values of the Draminckiestrus detector were ranged over 200-300 (means 254.86± 4.326). While, the values in cows affected with subclinical endometritis were 240-250 (means 247.14±1.844).There was no significant correlation between the age of the cows and both numbers of estrus, and values of estrus detector in the unaffected and affected cows. However, PH values of cervical mucus were significantly varied (P≤0.01) between the unaffected and affected cow, 7.271 ± 0.034 and 7.800 ± 0.0577, respectively. (Table 1).
Table 1: Shower correlated between Age, Estrus Number, Estrus detection, Endometrial Cytology PMN% and PH- value in unaffected cow compared with Sub-clinical endometritis cow.
| Groups Parameters | Unaffected cow | Sub-clinical endometritis cow |
P- value |
| Age\years | 5.00± 0.272 | 5.43± 0.841 | 0.084 |
| Estrus number | 2.80 ± 0.141 | 3.43 ± 0.369 | 0.550 |
| Estrus detection | 254.86± 4.326 | 247.14±1.844 | 0.436 |
| Endometrial Cytology PMNs% | 0.20± 0.080 |
14.86 ± 1.122** |
0.000 |
| PH- value |
7.271 ± 0.034 |
7.800 ± 0.0577** |
0.000 |
Values are mean ± S.E. ** mean significant at P≤0.01
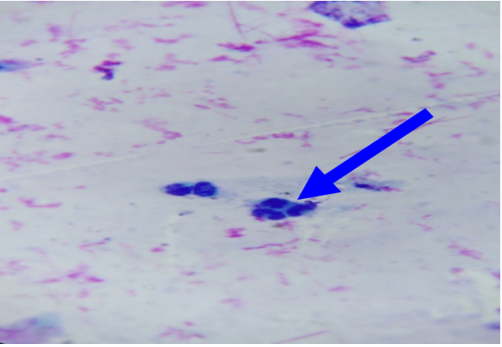
In unaffected cow, the percentage of endometrial PMNs ranged from 0-1% (mean 0.20±0.080); and 12-18% in cows affected with subclinical endometritis. There was a significant correlation between the increased age in the percentage of PMNs up to 12%≥ 18% in affected cows at (5.43± 0.841), forever there was a significant correlation in the detected subclinical endometritis by endometrial cytology indicated as an increasing percentage of PMNs up to 12%≥ 18% with the increased age of the affected cows at (5.43± 0.841). (Table 1, 2) (Figure 1, 2). The White side test was performed in the cervical mucus samples collected at estrus was recorded to change the mucus color to light yellowish, as well as observed in subclinical endometritis light yellow color compared with non-coloration in unaffected cows.
Penetration was measured using phase contrast microscopy. However the penetration can distinguish samples of frozen-thawed bovine semens were measured at penetrated ability of sperms for clear cervical mucus, whereas sperm
Table 2: Shower correlated between Age, Estrus Number, Estrus detection, Endometrial Cytology PMN%, PH- value White side test and Sperm penetration in unaffected cow compared with Sub-clinical endometritis cow.
| Correlations | Groups | Age\years | Estrus number | Estrus detection | Cytological method PMNs% | PH value | White side test | |
| Groups | r - value | 1 | .270 | .095 | -.123 | .977** | .718** | 1.000** |
| p - value | .084 | .550 | .436 | .000 | .000 | .000 | ||
| Age\years | r - value | .270 | 1 |
.494** |
-.160 |
.361* |
.374* |
.270 |
| p - value | .084 | .001 | .312 | .019 | .015 | .084 | ||
| Estrus number | r - value | .095 |
.494** |
1 | -.219 | .165 | .171 | .095 |
| p - value | .550 | .001 | .163 | .297 | .278 | .550 | ||
| Estrus detection | r - value | -.123 | -.160 | -.219 | 1 | -.126 | -.085 | -.123 |
| p - value | .436 | .312 | .163 | .428 | .590 | .436 | ||
| Cytological method PMNs% | r - value |
.977** |
.361* |
.165 | -.126 | 1 |
.745** |
.977** |
| p - value | .000 | .019 | .297 | .428 | .000 | .000 | ||
| PH value | r - value |
.718** |
.374* |
.171 | -.085 |
.745** |
1 |
.718** |
| p - value | .000 | .015 | .278 | .590 | .000 | .000 | ||
| White side test | r - value |
1.000** |
.270 | .095 | -.123 |
.977** |
.718** |
1 |
| p - value | .000 | .084 | .550 | .436 | .000 | .000 | ||
| Sperm penetration | r - value |
-1.000** |
-.270 | -.095 | .123 |
-.977** |
-.718** |
-1.000** |
| p - value | .000 | .084 | .550 | .436 | .000 | .000 |
.000 |
|
**Correlation is significant at the 0.01 level yellow color.
* Correlation is significant at the 0.05 level orang color.
motility is arrested by subclinical endometritis cervical mucus. It has been found that the majority of sperms died after a short distance of mucus penetration from cows affectedby subclinical endometritis while showed the majority of spermsmotility were penetrated and survived atthe mucus after moderate distance in unaffected cows.(Figure 3,4).
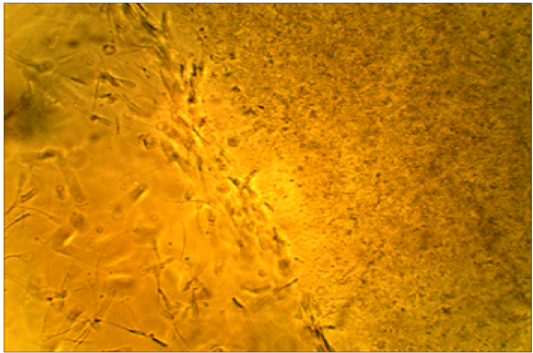
Figure 4: Died sperms after a short distance of mucus penetration in subclinical endometritis cow. X400.
Discussion
A previous study indicated the usefulness of utilizing the changes in the physico-chemical properties of cervical mucus for diagnosis of repeat breeder cow caused by sub-clinical endometritis (Kumar et al., 2015). In this study, the occurrence of subclinical endometritis was 16.66 %, which is consistence (17%) with Madoz et al. (2013). However, higher incidence rates were reported by Dubuc et al. (2010) and Carneiro et al. (2014) (18.7% and 26%, respectively). pH values of cervical mucus were significantly varied (P≤0.01) between the unaffected and affected cows (7.271 ± 0.034 and 7.800 ± 0.0577, respectively). Increase of pH in vaginal discharges higher than 7.8 is in agreement with a previous study in cattle affected with endometritis (Singla et al, 2004). This could be due to the presence of bacterial contamination in the uterine fluids where the increased pH is unsuitable for survival of spermatozoa and embryo in the uterus (Sheldon et al., 2006). The increased pH may results in metabolism of bacteria and inflammatory exudates in cervical mucus (Salphale et al., 1993). There was a significant correlation in the detection of subclinical endometritis by endometrial cytology indicated by an increased percentage of PMNs (up to 12%≥ 18%) with the increased age of the affected cows.This factor suggests that older cows showed an increased risk of subclinical endometritis. Many studies have stated that endometrial cytology isan effective technique for the initial detection of subclinical endometritis along with the microbial evaluation (Bajaj, 2015). However, the clear mucus discharge of ≥ 10 % polymorphonuclear cells (PMNs) are an indicator of subclinical endometritis, (Dutt et al., 2017). Kasimanickam et al. (2004) recorded that >18 % neutrophils count after 20-33 days postpartum, or>10 % neutrophils at 34-47 days postpartum in mucus samples as an indicative of subclinical endometritis; although, Gilbert et al. (2005) establish 5 % neutrophils at 40-60 days postpartum as an indicator of subclinical endometritis in cattle. While Barlund et al. (2008) used a neutrophil threshold value of 8% at 28-41 days postpartumin cattle to declare endometritis.
All the affected cows in this study were positive (100 %) for white side test, indicating positive for subclinical endometritis, where the coloration of the cervical mucus was changed to a light yellowish color, as compared with non-coloration in unaffected cows. This obviously indicated the ability of white side test in detection of subclinical endometritis, (Raja et al., 2012). Positive reaction to white side test might be due to neutrophil infiltration and metrorrhagia, which are usually seen during estrogen dominance (Ohtani et al., 1993). The normal cervical mucus contains fewer numbers of leukocytes to cause such changes in color, although in clinical and subclinical endometritis, cervical mucus contains a high number of leukocytes producing a color reaction (Pateria and Rawal, 1990). The sperm penetration test of frozen-thawed bovine semen was measured at penetration ability of sperms in clear cervical mucus, whereas sperm motility is arrested by subclinical endometritis cervical mucuson the worm slide at 37°C. It has been found that the majority of sperms died after a short distance of mucus penetration collected from cows affected with subclinical endometritis. In contrast,in unaffected cows, the majority of the penetrated and sperms were survived after moderate distancein the mucus. Cellular accumulation and leucocytes might limit the migration of spermatozoa in cervical mucus (Muzaffer, 2007) Therefore, it was recommended to utilize this test to distinguish between mucus collected from suspected subclinical endometritis in cows (Anilkumar and Devanathan, 1996). Presence of IgA–anti sperm antibodies can inhibit passage of spermatozoa through cervical mucus, prevent membrane fluidity changes needed for capacitation, and reduce the ability of spermatozoa to undergo the acrosome reaction, (Fijak and Meinhardt, 2006).
Conclusion
This study evaluated the using of different techniques for diagnosis of subclinical endometritis. In particular, for clinical practice, sperm penetration test was recognized as the most practical and simple method for detection of subclinical endometritis.
Acknowledgements
The project was approved by the scientific committee of Mosul University for animal research and animal welfare, and was fully supported by the Faculty of Veterinary Medicine of University of Mosul. The authors are indebted to the dairy cattles’ owners for their endlees colaboration.
conflict of interest
Authors declare no conflict of interest.
Authors Contribution
The authors themselves took the design of the study and collected the data and examined the cases and analyzed statistically and research writing
References