The Journal of Advances in Parasitology
Research Article
Ascaridia galli Challenge Model in Laying Hens
Nisha Sharma1,2*, Peter W. Hunt2 , Brad C. Hine2, Robert A. Swick1, Nishchal K. Sharma1, Isabelle Ruhnke1
1Animal Science, School of Environmental and Rural Science, University of New England, Armidale NSW 2351, Australia; 2F. D. McMaster laboratory, Chiswick, Commonwealth Scientific and Industrial Research Organisation (CSIRO), Armidale, NSW 2350, Australia.
Abstract | Background and objective: Ascaridia galli is one of the most prevalent helminths in free-range laying hens. This study was conducted to establish a reliable infection model forA. galli in laying hens. Materials and methods: A total of 20 Lohmann brown hens of 19 weeks age were assigned to 4 treatment groups (n=5 per group). Hens of group 1 were orally inoculated with 1000 A. galli eggs stored at 26°C, group 2 with 1000 A. galli eggs stored at 4°C and transferred to 26°C prior to inoculation. Hens were infected 3 times over a week period. Hens of group 3 were orally inoculated with 500 A. galli eggs stored at 26°C, 6 times over 2 week period. Hens in group 4 were infected with adult A. galli via cloaca. Intestinal immature worms were counted from 2 hens from each group after slaughter at 2 weeks post infection (p.i).Excreta was collected and analysed for A. galli eggs at 8 and 14 weeks p.i.. Blood was collected to examine A. galli specific antibodies and intestinal A. galli worms were counted at 16 weeks p.i.. Results: Hens in group 3 had the highest A. galli worm counts (P<0.001) after slaughter at 16 weeks p.i. compared to other groups. Excreta A. galli egg counts were highest in group 1 and 3 (P=0.02). Serum antibodies among the 3 orally infected groups was similar, but were higher than in hens of group 4 (P<0.01). Conclusion: Thus, The method either of inoculating hens orally with 500 A. galli eggs 6 times over 2 weeks period, or with 1000 A. galli eggs 3 times over a week period was the most reliable method tested.
Keywords | Infection methods, Inoculation, Nematodes, Parasites, Poultry.
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | October 04, 2017; Accepted | November 12, 2017; Published | November 20, 2017
*Correspondence | Nisha Sharma, Department Animal Science, University of New England, Armidale NSW 2351 Australia; Email: nsharma5@une.edu.au
Citation | Sharma N, Hunt PW, Hine BC, Swick RA, Sharma NK, Ruhnke I (2017). Ascaridia galli challenge model in laying hens. J. Adv. Parasitol. 4(3): 41-46.
DOI | http://dx.doi.org/10.17582/journal.jap/2017/4.3.41.46
Copyright © 2017 N-Sharma et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ascaridia galli is one of the most prevalent gastrointestinal parasites in free range laying hens (Gauly et al., 2007; Wongrak et al., 2014).The prevalence of this parasite, as revealed by several studies, ranges from 22-84% of total parasite load (Martin-Pachoet al., 2005; Sherwin et al., 2014). A. galli has a direct life cycle and thus the infection can spread among free ranging laying hens as they are constantly in close contact with excreta and soil (Permin and Hansen, 1998; Permin et al., 1999; Wongrak et al., 2014).The eggs of A. galli are resistant to the external environment (Tarbiet et al., 2015). Upon inoculation, the embryonated A. galli eggs hatch in the small intestine of their host. The released larvae can cause extensive destruction and erosion of the intestinal mucosa as well as proliferation of mucus secreting cells (Tugwell and Ackert, 1952; Ikeme, 1971). A. galli infections are often associated with reduced body condition, increased feed conversion ratio, and overall reduced health conditions (Chadfield et al., 2001). The infection may also act to supress the immune system of the host (Horning et al., 2003) thereby increasing the severity of concurrent diseases (Dahl et al., 2002; Permin et al., 2006).
In order to understand the epidemiology of A. galli and to work towards improved control strategies, experimental infections studies are necessary. Infection methods described to date have resulted in low establishment rates and subsequent worm burdens (Marcos-Atxutegi et al., 2009; Das et al., 2010) when compared with observations from natural infections (Permin et al., 1997; Kaufman et al., 2011).This highlights the need for an adequate infection study model using A. galli in laying hens. This challenge has been addressed in some studies through altering the conditions of infection models including the A. galli egg incubation temperature and humidity (Tarbiet et al., 2015), or using different incubation media (Permin et al., 1997). To address the varying resistance of A. galli eggs to the environmental conditions (Katakam et al, 2014), the influence of the age of the host (Gauly et al., 2005) and the hosts’ nutrition at time of infection have been investigated (Das et al., 2010). Therefore, the aim of this study was to establish a reliable infection model for A. galliin laying hens by optimising 1) the storage temperature after egg embryonation, 2) the inoculation frequency, 3) the numbers of A. galli eggs inoculated, 4) the parasite stage at inoculation and 5) the route of administration.
Materials and methods
Preparation of the Inoculation Material
In order to prepare embryonated A. galli eggs for artificial infection, mature A. galli nematodes were collected from the intestine of naturally infected laying hens after slaughter. The mature nematodes were washed in sterile phosphate-buffered saline (PBS), transferred into Roswell Park Memorial Institute (RPMI) media (with 0.1% 100 units/mL penicillin, 100 µg/mL of streptomycin, 250 ng/mL Amphotericin B) and cultured for three days at 37°C, changing the media every 24 hours. A. galli eggs that were shed into the media by the adult worms were collected from the medium by centrifugation after each 24 hour period. The concentrated A. galli eggs were resuspended in 0.1 N H2SO4 and kept at 26°C for up to six weeks. Embryonation of A. galli was judged to have occurred after 3 weeks of culture when fully formed nematodes were visible within the egg shell. For inoculation, the embryonated A. galli eggs were diluted into 0.05 M NaHCO3and then diluted in 0.05 M NaCl to generate the desired concentration of 500 or 1000 eggs/ml.
Ethical Approval
The research conducted was approved by the Animal Ethics Committee of the University of New England, Armidale, Australia (approval No AEC 14-089). Hens were individually housed in cages located at the University of New England and treated in accordance with the Model Code of Practice for the Welfare of Animals, Australia (CSIRO, 2002).
Experimental Design and Methods of Infection
A total of 20 Lohmann brown pullets of 19 weeks of age were randomly assigned to 4 treatment groups (n=5 per group). Hens were infected orally with A. galli embryonated eggs or cloacally with adult worms. Details of the infection model associated with each treatment group is shown in Table 1. Hens in group 4 were inoculated with adult worms through thec loaca within 4 hours after parasite collection from killed spent hens housed on a commercial farm. The adult worms were transported in RPMI media at 37°C and visibly alive at the time of cloacal transfer.
Table 1: Models of infection with A. galli eggs or adult worms (treatment groups)
| Details | Group 1 | Group 2 | Group 3 | Group 4 |
|
Mode of inoculation |
Oral | Oral | Oral | Cloacal |
|
Frequency of inoculation
|
3 times over 1 week | 3 times over 1 week | 6 times over 2 weeks | once |
| No. of embryonated eggs or adult worms inoculated | 1000 eggs | 1000 eggs | 500 eggs | 10 adult worms |
| Storage condition of eggs or adult worms prior to inoculation | 26˚C for >10 weeks | 4˚C for > 10 weeks post embryonation and then 26˚C for 2 weeks | 26˚C for 3 weeks | Room temperature |
Intestinal A. galli Worm Count
Two hens from each treatment group were sacrificed 2 weeks post infection (p.i.) to assess numbers of immature worms in the intestine by a method adapted from (MAFF, 1986). In short, the intestinal content was rinsed through a 1000µm metal sieve and collected on a 250µm sieve for immature worm counts by microscopy at 40x (Stereo compound microscope Olympus CX31, Tokyo, Japan). The remaining 3 hens of each treatment group were sacrificed at 16 weeks p.i. and intestinal immature and adult worms counted as described above.
Excreta Analysis for A. galli Egg Count
Fresh excreta samples were collected from 3 hens of each group at 8 and 14 weeks p.i. The number of A. galli eggs in each sample was estimated using a modified McMaster floatation method adapted from the MAFF, (1986) method. Briefly, four grams of excreta was placed in a 50 ml McMaster jar, 10 ml of water added, and the samples soaked for 30 minutes. Then, saturated NaCl solution was added to a total volume of 50 ml, stirred, and the suspension loaded onto McMaster egg counting chambers. A. galli eggs were counted by microscopy at 40x magnification (Stereo compound microscope Olympus CX31, Tokyo, Japan).
Detection of A. galli Specific Igy Antibodies in Hen Serum Using Elisa
A. galli worms were collected from spent hens at commercial farms after slaughter at 72 weeks of age to prepare antigen for the coating of ELISA plates. The blood was also
Table 2: A. galli worm counts in the intestine and their egg count in hen excreta
| Response |
Group 1 (1000 eggs 26˚C) |
Group 2 (1000 eggs, 4˚C) |
Group 3 (500 eggs, 26˚C) |
Group 4 (10 adults via cloaca) |
SEM |
P- value |
| Worm counts (2 weeks p.i.) | 0 | 0.33 | 12 | 0 | 3.12 | 0.06 |
| Worm counts (16 weeks p.i.) |
8ab |
1.66bc |
14.66a |
0d |
2.22 | 3.12 |
|
Excreta egg count (eggs/g 8 weeks p.i.) |
450 | 0 | 383 | 0 | 133 | 0.07 |
|
Excreta egg count (eggs/g 14 weeks p.i.) |
1850a |
266.66b |
916.66ab |
0c |
353 |
0.02 |
Values are least square means (n=5 per treatment group).a-bMeans in each column with different superscripts differ significantly (P<0.05)
collected from those hens then serum was extracted and stored at -20°C until further analysis. Female adult worms were separated and stored frozen at -80°C until processing. Approximately 3g of worms were mixed with PBS and homogenised with Ultra Turrax for 90 seconds, then centrifuged at 5820g for 30 min at 4°C. The protein concentration of the A. galli antigen preparation was determinedto be 5mg/ml using a Pierce BCA Protein Assay kit (Thermo Fisher-ScientificTM). The antigen prepared was partitioned into aliquots and stored at -80°C to be used to coat plates for the ELISA assay.
The following ELISA assay procedure was developed and validated as described (Norup et al., 2013) with slight modifications: Briefly, ELISA plates were coated with A. galli antigen extract (100µg/well), diluted to a final concentration of 1 µg/mL in carbonate buffer (pH 9.6) and incubated at 4°C overnight. Following incubation, the coating solution was flicked from the plates and wells blocked by adding 250 µl/well of blocking solution (PBS with 0.5% bovine serum albumin (BSA), pH 7.4), and incubated at room temperature for 2 hours. Plates were then washed 3times (PBS with 0.25% tween 20, 250uL/well), and test serum samples and control samples (positive and negative) were added to wells in the first column of the plate. Samples were then serially diluted in blocking solution (2 folds) across the plate to column 11 (total volume per well 100µL) and plates incubated for 1 hour at room temperature. Column 12 received only blocking solution and acted as a sample blank. Following washing (×5 times), goat anti chicken IgY antibody conjugated to horse radish peroxidase (Bio rad, Hercules, CA, USA) were added to the wells (100uL/well) and plates incubated for 1 hour at room temperature. Finally, plates were washed (×5 times, PBS with 0.25% tween 20) and 100µl/well of substrate solution (TMB-tetramethylbenzidine, Sigma, Aldrich, St. Louis, MO, USA) was added. Plates were incubated in the dark for 10-12 minutes and colour development then stopped with the addition of 50µL of 1M H2SO4. Colour development was quantified by reading the absorbance of individual wells at 450 nm with a 630 nm reference wave length. The raw titre values were calculated as the inverse of the dilution factor at which the absorbance of samples was predicted to reach a defined threshold.
In order to optimise the ELISA assay and to generate internal positive and negative control standards, serum samples from infected (free range) and non-infected (caged) hens from commercial farms were harvested. The blood samples was collected from laying hens before killing them at 16 weeks p.i. The serum was then extracted and stored at -20°C until required. Each step of the ELISA assay was optimised to minimise background signal and maximise the ratio of signal obtained from samples from infected versus non-infected birds. Positive and negative samples were then run on all plates to allow the performance of the assay to be monitored over time and to allow adjustments for plate to plate variation to be made.
Statistical analysis
The excreta A. galli egg count, intestinal A. galli worm count and IgY antibody titres of hen serum data were analysed by one-way ANOVA using Genstat (VSN International, 16th edition). For the parameters measured once, data were subjected to a one-way ANOVA to test the effect of treatments. For parameters measured over time, data were subjected to a two-way ANOVA with repeated measures. P-values < 0.05 were considered significant.
Results
Worm Counts
The mean number of immature and adult worms in the intestine of laying hens in each treatment group is presented in Table 2. The hens inoculated through the cloaca (group 4) had no A. galli worms present in the intestine at either 2 or 16 weeks p.i.. The hens orally inoculated with 500 eggs (group 3) tended to have higher worm counts (12 worms/hen) in the intestine two weeks p.i.. (P=0.06) compared to those orally inoculated with 1000 eggs, (0 worms/hen and 0.33 worms/hen in group 1 and 2, respectively). At 16 weeks p.i.., the hens in group 3 had significantly (P < 0.01) higher intestinal worm counts (14.66 worms/hen) than did hens in group 2 (1.66 worm/hen) but had similar (P > 0.05) worm counts to hens of group 1 (8 worms/hen).
Egg Counts
The mean number of A. galli eggs in the excreta of laying hens in each treatment group is presented in Table 2. The hens inoculated through cloaca (group 4) had no eggs present in the excreta at either 8 or 14 weeks p.i.. The hens inoculated with 1000 eggs (group 1) had similar egg counts (450 eggs/g and 1850 eggs/g at 8 and 14 weeks p.i.., respectively) to those infected with 500 eggs (group 3) (383 eggs/g and 916.66 eggs/gat 8 and 14 weeks p.i., respectively). Whereas hens inoculated with 1000 eggs (group 2) had lower egg counts (0 eggs/g and 267 eggs/g at 8 and 14 weeks p.i., respectively, P =0.02)
A. galli Specific Igy Antibody Titres in Hen Serum
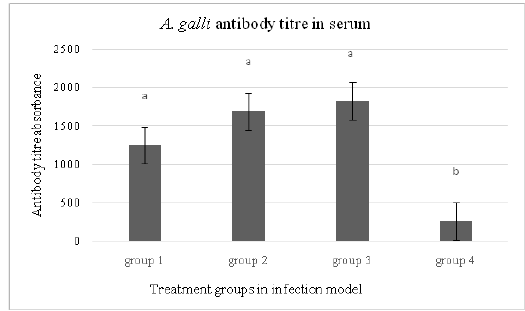
The mean A. galli specific IgY serum antibody titre of laying hens in each treatment group is presented in Figure 1. The mean serum antibody titre measured at 16 weeks p.i.was similar in all three orally infected treatment groups with values of 1200, 1600 and 1800 in groups 1, 2 and 3 respectively. The antibody titre was significantly lower in group 4 (200, P < 0.05) where the hens were infected via cloaca using the adult A. galli worms.
Discussion
Free range laying hens are allowed to roam freely in a restricted environment and thus the producers of free range eggs have less control over the environmental challenges birds are exposed to compared to other production systems, such as caged housing. The intact eggs of A. galli are composed of three layers: an inner permeable vitelline membrane, a resistant thick shell, and a thin albuminous outer layer (Ackert, 1931; Cruthers et al., 1974). These layers provide a high resistance of the A. galli egg to the external environmental conditions. A. galli eggs present in soil can complete their parasitic life cycle directly by excreta-oral transmission route, therefore it is important to investigate the impact of this parasite on the hen’s health and welfare in the free range production system and subsequent economic consequences. Results of the current study are expected to enable further research into the impacts of A. galli by developing a reliable infection model in laying hens.
Numbers of adult worms at 16 weeks p.i. observed in the intestine of hens did not differ between hens orally inoculated either with 500 A. galli eggs (group 3-14.66 worms/hen) or 1000 A. galli eggs (group 1- 8.00 worms/hen). These results are in contrast to the previous findings by Permin et al., (1997), where a higher number of worms were observed in the intestines of hens infected with single dose of 100 A. galli eggs as compared to 250 and 1000 A galli eggs. A previous study performed by Ackert and Herrick (1928) also observed a reverse dose dependency. By using A. galli eggs with the total number ranging from 25 to 500, Ackert and Herrick, (1928) found increased percentage of established larvae as eggs dosed decreased in number. Ikeme (1971a) observed that in hens infected with 1000 eggs/day, worm egg output was decreased, delayed and suppressed. He proposed that retarded egg development could be due to a density dependent phenomenon driven by worm to worm interactions, or may be related to host immunity. Herd and McNaught (1975) found a higher degree of antigenic stimulation and immune reaction in New Hampshire and White Leghorn chickens when exposed to a higher number of A.galli nematodes. Similar to studies perfomed by (Martin-Pacho et al., 2005, Marcos- Atxutegi et al., 2009; Schwarz et al., 2011), we observed a rise in A. galli specific serum IgY in all 4 treatment groups. However, in our experiment, there was a similar rise in A. galli specific antibody IgY titre in the serum for all hens orally dosed (groups 1, 2, 3) but not for group 4 where cloacal insertion of adult worms was undertaken. A.galli nematodes are expected to induce strong T-helper (Th2) responses in the host since this parasite is considered as a strong Th2 driving pathogen (Degen et al., 2005; Schwarz et al., 2011).Th2 cells play an important role in immune responses against helminths and extracellular parasites (O’Garra, and Arai, 2000). Presence of antibodies in group 4, in the absence of an established infection may indicate that the hens had been previously exposed to the parasite, such as during their time at the commercial rearing facilities. It is suggested to use un-infected control group to validate the infection model in the future trials so as to assess influence of background responses on results.
Hens in group 3 which were orally inoculated 6 times over a 2 week period had higher worm counts than other groups suggesting inoculation might have an effect on the establishment of infection. Hens can develop natural resistance to A. galli infections, evidenced by the fact that after infections, only a small percentage of A.galli worms survive and establish in the host. It is also possible that A. galli eggs encountered may be non-viable and therefore break the life cycle. Ackert and Herrick (1928) suggested that large single infections in hens can result in increased resistance to infection. However; as reported by Sadun (1949), a single oral dose of 14,000 embryonated eggs per birds can be lethal. In contrast to methods used previously, in our experiment, the number of eggs were divided into multiple doses which were applied three times over a 1 week period (groups 1 and 2) or six times over a two weeks period (group 3).
The storage temperature for A. galli eggs used to infect birds in group 1 and 3 was 26°C which is thought to be optimal for embryonation (Permin, and Hansen, 1998). Rahimian et al. (2016) found that eggs stored at 20-30°C can develop to infective L3 stage after 7-21 days of incubation and remain viable at this temperature. In group 2, A. galli eggs were kept at 4°C for more than 10 weeks post embryonation and then transferred to 26°C for 2 weeks prior to inoculation. Infection establishment was significantly reduced in these birds, as evidenced from excreta analysis (0 eggs/g on 8 weeks and 266 egg/g on 14 weeks p.i.) and worm counts (0.33, and 1.66 on 2 weeks and 16 weeks p.i.), indicating that cold storage of embryonated eggs is detrimental to their ability to establish a subsequent infection.
Following infection, the pre-patent period for Ascaridia galli has been reported to be 5-8 weeks (Kerr, 1955). In the current study, hens of treatment groups 1 and 3 initiated egg shedding at 8 weeks p.i. (450 eggs/g, 383 eggs/g, respectively). In addition, the excreta analysis for A. galli egg counts 8 weeks p.i. and 14 weeks p.i. was similar in both groups. In other species such as pigs, Christensen et al. (1995), a lower excretion rate of parasites eggs in the groups given a higher infection dose with Oesophagostomum dentatum have been observed when the higher infection dose was 10 times that of the lower infection dose whereas in the present study, excreta egg counts were similar in birds when the infection dose was doubled. The absence of immature worms and adult worms observed in hens from group 4 suggests that infection through insertion of adult worms through the cloaca is not a viable alternative to oral administration of embryonated eggs.
In conclusion, the findings of this study suggest that the infection model applied to hens in treatment group 1 (1000 A. galli eggs stored at 26°C inoculated over a week period) or treatment group 3 (500 A. galli eggs stored at 26°C inoculated over a two week period) were both effective in establishing infection and as such are both expected to provide a reliable model of A. galli infection in laying hens. Cold storage of embryonated eggs is not conducive to subsequent infection, nor was administration of mature worms via the cloacal route. The ELISA method established to detect A. galli specific antibody IgY is expected to be able to detect infection in birds but the relationship between antibody level and infection intensity and status will require further research for validation.
Acknowledgements
We would like to thank the Poultry CRC, Australia for providing funding for this trial, the Wiseman family for donating spent hens, Jody Mc Nally and Amy Bell for technical assistance with ELISA tests.
Conflict of interest
There are no conflicts of interest.
Authors Contribution
Experimental design and ideas developed by Isabelle Ruhnke, Peter W Hunt and Brad C Hine. Data and samples collection by Nisha Sharma and Nishchal Sharma. Lab analysis by Nisha Sharma. Data analysis helped by Peter W hunt. Manuscript preparation by Nisha Sharma. All co-authors revised and commented on the manuscript.
References