The Journal of Advances in Parasitology
Research Article
Prevalence of Congenital Toxoplasmosis in Pregnant Women with Complicated Pregnancy Outcomes in Assiut Governorate, Egypt
Ahmad Mouhamad Mandour1, Mahmoud Elhady Mouhamad Mounib1, Hanan ElDeek Mouhamad Eldeek1, Alzahraa Abdel Raouf Ahmad1*, Abdel Rahman Mahmoud Mouhamad Abdel Kader2
1Parasitology Department, Faculty of Medicine, Assiut University, Egypt; 2Obstetrics and Gynecology Department, Faculty of Medicine, Assiut University, Egypt.
Abstract | Toxoplasma gondii is an intracellular protozoan that can infect all mammals including man. It causes congenital infections in humans. Knowledge of its prevalence in pregnant women would be a valuable approach for planning appropriate preventive strategies. Meanwhile early diagnosis of toxoplasmosis in pregnant women is necessary to get effective treatment and prevent fetal complications. The current cross–sectional study aimed to determine the rate of T. gondii infection and maternal-fetal transmission in high risk pregnant women with complicated pregnancy outcomes in Assiut Governorate, Egypt. Out of 182 pregnant women who were screened for Toxoplasma-specific IgG and IgM antibodies with ELISA, 125 samples (68.7%) were seropositive. Seventy-three samples were IgG seropositive (IgG+/IgM−), showing that 40.1% of subjects were immune to Toxoplasma infection. Fifty-seven samples were seronegative (IgG-/ IgM-) meaning that 31.3% of subjects were susceptible to primary infection. The rate of probable acute Toxoplasma infection was 28.6% in all participants. (IgG+/IgM+ & IgG-/IgM+ were 13.2% & 15.4% respectively). Significant relations were found between Toxoplasma-specific IgG and residency in rural areas, consumption of milk/milk products, contact with soil, and eating undercooked meat or viscera. In conclusion, we reported high prevalence of T. gondii infection among pregnant women with adverse pregnancy outcomes associated with trans-placental transmission of infection in Assiut governorate, Egypt. Sources of infection revealed herein might represent potential threats for primary infection in seronegative women.
Keywords | Toxoplasma gondii, Pregnancy, Women, Prevalence, Egypt
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | December 02, 2016; Accepted | January 13, 2017; Published | January 20, 2017
*Correspondence | Alzahraa Abdel Raouf Ahmad, Parasitology Department, Faculty of Medicine, Assiut University, Egypt; Email: zahraahassan2009@gmail.com
Citation | Mandour AM, Mounib MEM, Eldeek HEM, Ahmad AAR, Abdel-Kader ARMM (2017). Prevalence of congenital toxoplasmosis in pregnant women with complicated pregnancy outcomes in Assiut governorate, Egypt. J. Adv. Parasitol. 4(1): 1-8.
DOI | http://dx.doi.org/10.14737/journal.jap/2017/4.1.1.8
ISSN | 2311-4096
Copyright © 2017 Mandour et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The protozoan T. gondii is an obligate intracellular parasite that infects humans and a broad spectrum of vertebrate hosts (Skariah et al., 2010). It causes toxoplasmosis which is one of the most common parasitic infections in humans and is most typically asymptomatic. However, primary infection in a pregnant woman can cause severe and disabling disease in the developing fetus including abortion (Gebremedhin et al., 2013), fetal death, or neurological or ocular damage of the fetus (Tenter et al., 2000).The severity of clinical disease in congenitally infected infants is related inversely to the gestational age at the time of primary maternal infection (Robbins et al., 2012; McAuley, 2014).
In fact serological screening tests concerning detection of the prevalence of T. gondii infection in pregnant women and the incidence of acute maternal toxoplasmosis in different populations is a valuable epidemiologic tool to estimate the amplitude of congenital toxoplasmosis in pregnant women. It also helps to estimate the socioeconomic costs of this infection and to evaluate the potential benefits of screening programs (Swai and Schoonman, 2009).
Worldwide, many serological surveys had been conducted on the seroprevalence rate of T. gondii in pregnant women, women of reproductive age or in the general population and they showed a considerable variation from 7.5 to 95% in different parts of the world (Asthana et al., 2006). Toxoplasma prevalence is evolving continuously and varies greatly among different countries and geographical areas within the same country as well. It is affected by different and complex risk factors whether environmental, socioeconomic and health related practices (Pappas et al., 2009). The awareness of these risk factors may determine prevention and control strategies and the optimal health policies that could be designed in different communities (Remington et al., 2001; Montoya and Liesenfeld, 2004).
In Egypt, several studies have been performed to detect T. gondii infection in human and animals (Elsheikha et al., 2009; Ibrahim et al., 2009; Ghoneim et al., 2010; El Deeb et al., 2012; Nassef et al., 2015). However, the data describing the role of T. gondii infection in acquiring serious adverse pregnancy outcomes as abortion, stillbirths or congenital malformation among pregnant women in Egypt is still limited (Ibrahim et al., 2009).
The present study aimed to estimate the seroprevalence rate of T. gondii infection in high risk pregnant women with bad obstetric outcomes attending Women’s Health Hospital at Assiut University. Also, the research aimed to correlate the seropositivity rate to some potential risk factors of T. gondii among the study subjects.
Materials and methods
Study Setting
The present study is a descriptive analytical cross-sectional study. The samples were collected from Women’s Health Hospital at Assiut University during the period from September 2013 to March 2014.
Target Population
The subjects included in the study were pregnant females with unexplained bad obstetric outcomes and were chosen according to certain eligibility criteria justified by a formulated questionnaire according to similar published researches (El Deeb et al., 2012) and investigations performed for each patient in the hospital records. The questionnaire (placed at the end) included; the basic socio-demographic data, obstetrical history, general knowledge about toxoplasmosis, lifestyle issues and other risk factors that may be relevant to Toxoplasma infection, and eligibility criteria for choosing the subject groups as shown in the appendix.
Subject Groups
A total of one hundred eighty two (182) cases were chosen for the study and categorized into four subject groups:
1st group: included female cases with abortion (whether first trimester or second trimester abortion) without any obvious cause.
2nd group: included cases with unexplained intrauterine fetal death (IUFD).
3rd group: included cases with unexplained preterm labour.
4th group: included cases with congenitally malformed newborns (CMF)
Serology
From each subject included in the study, 3 ml of venous blood was collected under aseptic precautions. Serum was separated from whole blood by centrifugation at 3,000 rpm for 5 min, labeled, and kept in coded Eppendorffs at -20˚C for further serological testing. Each serum sample was tested for the presence of anti-Toxoplasma IgM and IgG antibodies using commercial TOXOPLASMA GONDII ELISA kit (BioCheck, Inc., Foster City, USA) following the manufacturer’s instructions.
The results were read by optical density at 450 nm on an ELISA reader. The results interpreted for IgG and IgM antibodies as follows: The sample is considered: Positive: if the ratio is > 1.00 or greater, equivocal: > 0.91 - < 0.99, negative: if the ratio is < 0.90.
Statistical Analysis
Statistical analysis was done with software (SPSS version 20). Test of significance as chi-square and Fisher exact test were used to detect the relationship between qualitative variables. Statistical significance was defined as a P-value of <0.05.
Results
Basic Socio-Demographic Data of the Studied Participants
As regards age and residence, it was found that the age groups of the participants were ranging from 17-43 years old (Mean of age = 26.4+ SD 6.0). The most prevalent age group was less than 25 years old (43.4 %). On the other hand, it was found that 52.2% of the participants were from rural areas (n= 95) while those from urban areas represented 47.8%. (Table 1).
As regards gravidity and gestation, the multigravida females were the prevalent ones in the study with a percentage of 72.5% (n=132). Most of the participants were in the third trimester of pregnancy (40.1%). However, most cases were belonged to abortion group (94 cases, 51.7 %), with 41.5% recurrence rate (Table 1).
Table 1: Correlation between socio-demographic characteristics and Seropositivity for T. gondii in pregnant women included in the study
|
Total (182) No. (%) |
ELISA results |
P. value |
||||
|
Positive (n=121) |
Negative (n=61) |
|||||
|
No. |
% |
No. |
% |
|||
|
Age categories |
||||||
|
< 25 years |
79 (43.4) |
53 |
67.1 |
26 |
32.9 |
0.863 |
|
25 - 30 years |
57 (31.3) |
39 |
68.4 |
18 |
31.6 |
|
|
> 30 years |
46 (25.3) |
33 |
71.7 |
13 |
28.3 |
|
|
Residence |
||||||
|
Rural |
95 (52.2) |
73 |
76.8 |
22 |
23.2 |
0.013* |
|
Urban |
87 (47.8) |
52 |
59.8 |
35 |
40.2 |
|
|
Gestation |
||||||
|
First trimester |
66 (36.3) |
45 |
68.2 |
21 |
31.8 |
0.848 |
|
Second trimester |
43(23.6) |
28 |
65.1 |
15 |
34.9 |
|
|
Third trimester |
73 (40.1) |
52 |
71.2 |
21 |
28.8 |
|
|
Parity |
||||||
|
primigravida |
50(27.5) |
35 |
70 |
15 |
30 |
0.813 |
|
multigravida |
132(72.5) |
86 |
65.2 |
46 |
34.8 |
|
|
Patients groups |
||||||
|
Abortion |
94 (51.7) |
66 |
70.2 |
28 |
29.8 |
0.291 |
|
IUFD |
30 (16.5) |
21 |
70.0 |
9 |
30.0 |
|
|
preterm |
43 (23.6) |
31 |
72.1 |
12 |
27.9 |
|
|
CMF |
15 (8.2) |
7 |
46.7 |
8 |
53.3 |
|
*: Statistically significant difference; P. value < 0.05
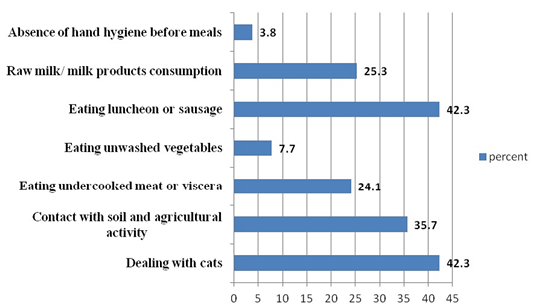
Regarding to Toxoplasma possible risk factors, there were some factors that showed relatively high representation as dealing with cats, contact with soil and agricultural activity, and eating luncheon or sausage (42.3%, 35.7% and 42.3%, respectively). In contrast with other risk factors included in the study, raw milk consumption, eating undercooked meat or viscera and eating unwashed vegetables were less represented (25.3%, 24.1% and 7.7%, respectively). Absence of hand hygiene showed the lowest representation in the study (3.8%) as shown in Figure 1.
Toxoplasma Seroprevalence Rates
The seroprevalence for T. gondii infection in high risk pregnant women included in the study was found to be 68.7% (n=125). The seropositivity rates for anti-Toxoplasma IgG and IgM were shown in Figure 2.
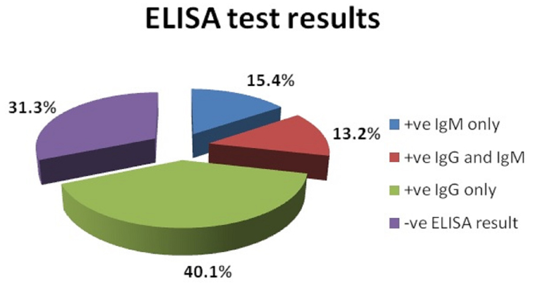
Figure 2: The percentage of Toxoplasma specific IgM and IgG antibodies in the sera of pregnant women included in the study
The high prevalence of T. gondii seropositive cases was observed in age group more than 30 years old, primigravid females, females at the third trimester of pregnancy and preterm labor cases. However, there wasn’t any significant association observed between seropositive and seronegative cases in the different comparative groups (Table 1).
Table 2: Correlation between some relevant risk factors and sero-positivity for T. gondii in pregnant women included in the study
|
ELISA Results |
P-value |
||||
|
Positive |
Negative |
||||
|
No. |
% |
No. |
% |
||
|
Dealing with cats |
|||||
|
Yes |
52 |
67.5 |
25 |
32.5 |
0.775 |
|
No |
73 |
69.5 |
32 |
30.5 |
|
|
Contact with soil |
|||||
|
Yes |
59 |
76.6 |
18 |
23.4 |
0.047* |
|
No |
66 |
62.9 |
39 |
37.1 |
|
|
Eating undercooked meat or viscera |
|||||
|
Yes |
39 |
88.6 |
5 |
11.4 |
0.001* |
|
No |
86 |
62.3 |
52 |
37.7 |
|
|
Eating unwashed vegetables |
|||||
|
Yes |
9 |
64.3 |
5 |
35.7 |
0.945 |
|
No |
116 |
69.0 |
52 |
31.0 |
|
|
Eating luncheon or sausage |
|||||
|
Yes |
52 |
67.5 |
25 |
32.5 |
0.775 |
|
No |
73 |
69.5 |
32 |
30.5 |
|
|
Raw milk/milk products consumption |
|||||
|
Yes |
37 |
80.4 |
9 |
19.6 |
0.046* |
|
No |
88 |
64.7 |
48 |
35.3 |
|
|
Absence of hand hygiene before meals |
|||||
|
Yes |
5 |
71.4 |
2 |
28.6 |
0.873 |
|
No |
120 |
68.6 |
55 |
31.4 |
|
*: Statistically significant difference P. value
Meanwhile, there was a significant T. gondii seropositivity in pregnant women living in rural areas, had contact with soil and farm animals or had agricultural activates, consumed raw/undercooked meat or viscera, and consumed raw milk or other milk products (Table 1 and 2).
Discussion
The major finding in this cross sectional study showed that the overall sero-prevalence of toxoplasmosis in the screened pregnant women was as high as 68.7%. The rate of probable acute Toxoplasma infection was 28.6% in all participants. 40.1% of subjects were IgG seropositive indicating that they were immune to Toxoplasma infection (chronic toxoplasmosis). On the other hand, 31.3% of subjects were seronegative meaning that they were non-immune and susceptible to primary infection in next pregnancy.
The high prevalence rate of Toxoplasma infection in the present study agreed with similar previous studies performed in Egypt reporting the prevalence of toxoplasmosis in complicated high risk pregnant women who screened by ELISA test as described in Table 3. Furthermore, there are many other studies conducted on pregnant females during the antenatal care whether symptomatic or asymptomatic to detect the prevalence of T. gondii infection in antenatal population and the risk of transmission of infection to the fetus. These studies showed a wide variation in the prevalence ranged from 22% to 67.5% (Shatat et al., 2006; El Deeb et al., 2012).
As regards to the nearby Arabian countries; the sero-prevalence rate of toxoplasmosis that has been reported in several studies showed also a considerable variation ranging from 20% to 62.2% in these countries and within the same country as well (Aqeely et al., 2014) (Table 3). Moreover, the prevalence of toxoplasmosis was found to be highly variable in different countries worldwide where it ranged from 4.1% to 81.4% based on the geographical region (Gebremedhin et al., 2013).
From previous data, it was recognized that there is a wide range of variation in the prevalence of toxoplasmosis in Egypt and other Arabian countries which could be attributed to the use of different serological techniques and /or kits with different rates of sensitivity and specificity (Tammam et al., 2013). Furthermore, it should be taken into consideration that data inferred from recent reports, whether nationally or in nearby countries, should be interpreted with caution due to the small sample size and insufficient data regarding the characters of the studied populations or relevant Toxoplasma risk factors. Moreover, the different geographical and environmental conditions favoring transmission of infection influence this variation (Montoya, 2002; Nijem and Al-Amleh, 2009). The increased rate of seropositivity over the past 20 years suggested a wide range of exposure to T. gondii and the increased transmission rate of infection in these areas (El Deeb et al., 2012).
Table 3: The sero-prevalence of T. gondii in different governorates of Egypt and some Arabian countries
|
Country |
Seroprevalence (%) |
Reference |
|
|
Egyptian governorates |
Assiut |
55.8% |
Abdel-Rahman et al. (2004) |
|
Menoufia |
52.2% |
Nassef et al. (2015) |
|
|
El Minia |
50.8% |
Kamal et al. (2015) |
|
|
Qina |
46.1% |
Tammam et al. (2013) |
|
|
Qualyobia |
44.7% |
Hussein et al. (2001) |
|
|
Some Arabian countries |
Sudan |
34.1% |
Elhag and Elturabi (2015) |
|
28.4% |
Elnahas et al. (2003) |
||
|
Saudi Arabia |
20% |
Aqeely et al. (2014) |
|
|
29.4% |
Al-Harthi et al. (2006) |
||
|
Iraq |
38.4 % |
Mohammad et al. (2013) |
|
|
Yemen |
41.90 % |
Al-Nahari and Al-Tamimi (2010) |
|
|
Lebanon |
62.2% |
Bouhamdan et al. (2010) |
|
|
Palestine |
27.9% |
Nijem and Al-Amleh (2009) |
|
|
Qatar |
29.8% |
Abu-Madi et al. (2008) |
|
|
Morocco |
50.6% |
El Mansouri et al. (2007) |
|
|
Kuwait |
53.1% |
Iqbal and Khalid (2007) |
|
|
Bahrain |
21.8% |
Tabbara and Saleh (2005) |
In the present study, we studied the correlation between T. gondii prevalence rate in the studied groups of pregnant women and their socio-demographic characteristics. It was found that older participants whose age more than 30 years old showed the highest positivity rate (71.7%). However there was no significant association between an increasing maternal age and Toxoplasma sero-prevalence as reported by other authors (Elsheikha et al., 2009; Alvarado-Esquivel et al., 2011). The relative increase in Toxoplasma sero-positivity with increasing age may be attributed to the cumulative effect of exposure to the infective stages of the parasite and the relative increase in sources of infection to which women are subjected (Gebremedhin et al., 2013). This observation coincides with another finding in the study which is increased percentage of multiparous women with complicated pregnancy outcomes than primigravid females (72.5% in comparison with 27.5%).
Moreover, living in rural communities was found to be significantly associated with T. gondii infection. These results were in agreement with other studies (El-Gozamy et al., 2009; Fouladvand et al., 2010; Tammam et al., 2013). The high positivity (76.8%) for toxoplasmosis in rural areas of Assuit governorate may reflect the life style and hygienic behavior of the population in these rural areas which make them more susceptible to the infection.
The high possibility of infection in rural than urban areas may be attributed to high density of domestic animals in rural areas as well as the favorable environmental conditions for T. gondii oocysts to sporulate, contact with farm animals, lack of sanitary water, and the habit of eating unwashed vegetables or fruits (El Deeb et al., 2012). It was found that rural communities in developing countries have a considerable epidemiological importance as they have high rate of infection, lack of health care services and deficiency of diagnostic facilities for infectious diseases (Alvarado-Esquivel et al., 2009). So, future studies will be strongly warranted in rural areas.
Actually, knowledge of T. gondii sero-prevalence in pregnant women in conjunction with possible risk factors of acquiring the infection could be a useful tool for planning appropriate control and preventive strategies (Swai and Schoonman, 2009). In the present study, the association between T. gondii sero-prevalence and the presence of some susceptible risk factors for T. gondii infection has been detected. There was a significant relationship Toxoplasma seropositive cases and residency in rural areas, consumption of milk or dairy products, contact with soil, and eating undercooked meat or viscera. These results are in agreement with other previous studies (Cook et al., 2000; Tekay and Ӧzbek, 2007; Elsheikha et al., 2009; Deji-Agboola et al., 2011; El Deeb et al., 2012; Gebremedhin et al, 2013).
Contact with soil and farm animals and consumption of unpasteurized milk and/or milk products as locally known “Kareish cheese” were obviously related to poor hygienic practice of the studied population (Elsheikha et al., 2009; El Deeb et al., 2012). Such practice is noteworthy related to infection acquisition. In our community, the low socioeconomic status and low educational level of wide scale of population in rural areas in Upper Egypt, contribute to high prevalence of toxoplasmosis in these areas.
Recent studies highlighted the role of sheep in the transmission of toxoplasmosis to humans (Zhang et al., 2016). In Egypt previous studies showed that there is high sero-prevalence for toxoplasmosis in sheep and goat (Barakat et al., 2009; Ghoneim et al., 2010). These findings correlated eating raw infected sheep and goat meat or drinking their raw milk with increased Toxoplasma infection in pregnant women. Also, the preferred habit of eating some traditional Egyptian meat meals such as “Hawawshi”, “Shawerma”, kebabs, core pastrami and brain tissue may contribute to increase risk factors for toxoplasmosis in Egypt (Abd El-Razik et al., 2014). Other studies showed different findings from ours with no relationship between eating raw or undercooked meat and viscera and increased risk of Toxoplasma infection (Ertug et al., 2005; El Deeb et al., 2012). They attributed the lack of association due to low consumption of meat due to its high cost in addition to preferred well-done meat by locals.
Although contact with cat was a well-known risk factor (Spalding et al., 2005; Elsheikha et al., 2009), we did not find a relationship with Toxoplasma infection. Similarly, other reports ruled out contact with cats as a risk factor (Alvarado-Esquivel et al., 2009; Fouladvand et al., 2010; Deji-Agboola et al., 2011).
In fact Egyptian communities showed abundance in stray cats with 97.4% of Toxoplasma prevalence in feral cats, indicating high environmental contamination with Toxoplasma oocysts (Al-Kappany et al., 2010). However, the lack of association with direct cat contact should be explained in the presence of other risk factors as; contact with soil, eating unwashed raw vegetable or fruit and poor hand hygiene. This is due to potential contamination with sporulated oocysts in soil rather than on cat fur (Dubey, 2000).
Furthermore, the present study could not identify a significant association between eating luncheon or sausage and the risk of acquiring T. gondii infection as observed by other researchers (Alvarado-Esquivel et al., 2007; Elsheikha et al., 2009). This may be attributed to the preference of fresh meat than industerized meat products by locals.
Conclusion
In conclusion, we report high prevalence for T. gondii infection among pregnant women with adverse pregnancy outcomes in Assiut Governorate. The significant risk factors presented herein might represent potential sources of infection to seronegative women. Accordingly, tracking sources of Toxoplasma infection in the environment or food sources and implementation of health education programs is highly recommended as an appropriate preventive strategy. Also, the present study emphasizes the need for further studies on national level to reveal rates of T. gondii infection, maternal–fetal transmission as well as potential risk factors.
Ethical Approval
All study subjects were informed about the study and informed consents were obtained from all women. Ethical clearance was obtained from the Ethical Committee, Faculty of Medicine, Assiut University.
Acknowledgements
The authors thank Assiut University Hospitals in Assiut, Egypt for the support and offering the use of its facilities for this project. The project was funded by Assiut University Faculty of Medicine.
Conflict of interest
Authors declare that there is no conflict of interest
Authors’ Contribution
AlZahraa Abdel Raouf Ahmad and Hanan ElDeek Mouhamad ElDeek initiated the idea, made the study design, statistical analysis, interpretation of results, wrote and and revised the manuscript. Ahmad Mouhamad Mandour and Mahmoud ElHady Mouhamad Mounib revised the manuscript. Abdel Rahman Mahmoud Mouhamad Abdel Kader shared in study design and sample collection.
References