The Journal of Advances in Parasitology
Research Article
Prevalence of Fasciolosis in Small Ruminants and Associated Risk Factor in and Around Kombolcha
Ashenafi Kiros1*, Birhan Tadesse1, Alemu Aylate1, Biniam Tadesse2
1School of Veterinary, Wolaita Sodo University, Wolaita, Ethiopia; 2Ethiopian Veterinary Drug and Feed Adminstration and Control Authority.
Abstract | A cross sectional study was conducted from November 2007 to April 2008 to determine the prevalence of ovine fasciolosis in the field in and around kombolcha. A total 500 faecal samples comprising of 395 sheep and 105 goats were randomly selected and subjected to coprological examination by sedimentation technique. The sampling method has involved all age group, sex and three ago-ecological zones. Out of the total examined feacal 305 samples were found positive for fasciolosis with an overall prevalence rate of 61 per cent. Prevalence variation was existed between the study woredas, with the highest being at Harbu woreda (71.19%) followed by mitikollo (61.64%), charosa (58.88%) and lastly kedida (58.51%) at Kallu woreda. Prevalence of fasciolosis in ovine species was drastically higher than caprine species. There was no statistical difference between male and female, local and cross breed animals. However, adult and poor condition sheep and goats were noticeably susceptible to the disease. Fasciolosis was a disease of prime concern in two woredas of south wollo zone bordering Borkena River and that it must be remarked in priority list in any animal control program to be envisaged in the region. Strategic anthelminthic treatment with appropriate flukicidal drugs should be practiced two times a year.
Keywords | Shoat, fasciollosis, prevalence, sedimentation anthelminthic
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | January 26, 2016; Revised | March 23, 2016; Accepted | March 28, 2016; Published | April 16, 2016
*Correspondence | Ashenafi Kiros, School of Veterinary Wolaita Sodo University, Wolaita, Ethiopia; Email: nafikw@gmail.com
Citation | Kiros A, Tadesse B, Aylate A, Tadesse B (2016). Prevalence of Fasciolosis in small ruminants and associated risk factor in and around Kombolcha. J. Adv. Parasitol. 3(2): 61-65.
DOI | http://dx.doi.org/10.14737/journal.jap/2016/3.2.61.65
ISSN | 2311-4096
Copyright © 2016 Kiros et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Fasciolosis is an economically important disease of domestic livestock, in particularly cattle, sheep, goats and occasionally man. The disease is caused by digenean trematodes of the genus Fasciola commonly referred to as liver fluke. The two species most commonly implicated as the etiological agents of fasciolosis are Fasciola hepatica or temperate liver fluke and F. gigantica or tropical liver fluke (Mas-coma et al., 2005). The ability of Fasciola to spread is related to the capacity of fasciolids to colonize and adapt to new environments, new definitive hosts as well as intermediate hosts (Chhabra and Singla, 2009).
Small ruminant fasciolosis due to F. hepatica and F.gigantica is endemic and economically important in many parts of Ethiopia with prevalence ranging from 11.5% to 87.0% (Abdulhakim et al., 2012). In central Ethiopia, the annual loss due to ovine fasciolosis was estimated to be 48.4 million Ethiopian Birr of which 46.5, 48.8 and 4.7% were due to mortality, loss of productivity and liver condemnation respectively (Ahmed et al., 2007).
Knowledge on the prevalence of the small ruminant parasite is crucial for strategic prevention and control. Despite the presence of large number of small ruminants in and suitable environment for the fasciollosis in Amahara region in general and in Kombolcha in particular, there is scanty information on the study of fasciolosis in small ruminants.
Therefore, the objective of the study was to determine the prevalence of fasciolosis and to contemplate its associated risk factors in and around kombolcha fields by conducting coprological investigation on shoat feacal sample from field.
Materials and methods
Study Area
The study was conducted in and around kombolcha zone which is 375 km north east from Addis Ababa at of an altitude of 1500 -1840m above sea level. The woreda receives an annual rainfall of on average 750 – 900 mm with an annual mean temperature of 11.70C (minimum) and 23.90C (maximum). The woreda falls in to three agro climatic zones: Low lands (kola) = 52 %, Medium high lands (weyna dega) =34% and High lands (Dega).
Study Animals and Sample Size
The study animals comprised sheep and goats at the field areas of Kombolcha.
Sheep and goats kept in both semi-intensive system farms and under extensive production system was included in the study. The desired sample size for the study is calculated using the formula given by (Thrusfied, 2005).
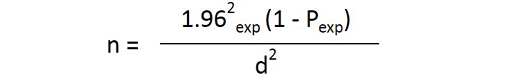
Where n = required sample size, Pexp = expected prevalence, d = desired absolute precision, 1.96 = z- value for the 95% confidence level
With 95% confidence interval and at 5% absolute precision and there is no previous studying was conducted in and around kombolcha so the prevalence of small ruminant fasciolosis was considered at 50%. Based on these information 500 samples from sheep and goats were examined respectively.
Study Design and Sampling Methodology
A cross sectional study was conducted from November 2007 to April 2008 to determine the prevalence of ovine fasciolosis by coprological investigation on shoat in field in and around kombolcha. In this study, the area was classified in to three based on altitude and climatic conditions: lowland, midland and highland. Representative districts (woredas) which contribute certain peasant associations were purposively selected by considering their altitudes.
Sampling and Coprological Examination
The faecal samples for parasitological examination were collected directly from the rectum of each animal with hygienic condition; faecal samples were collected from all groups of animals on the day of allocation. The time of collection of faecal samples was uniformly maintained throughout the study period to avoid possible diurnal variations in fluke egg output (Roy and Sunkhla, 1971). The samples were collected with clean universal bottles preserved with 5% formalin and each sample was clearly labeled with permanent marker about the information’s like animal identification (species, age, breed of animal), date and place of collection. Samples were packed and dispatched a cool box to avoid the eggs developing and hatching. In the laboratory, coprological examination was performed to detect the presence of fasciola eggs using the standard sedimentation technique.
Sedimentation Technique
From collected samples for each case 3gms of feaces was measured and put in to a mortar. Then 42ml of ZNSO4 (zinc sulphate) solution as a floating medium will be added and crushed thoroughly with a pistle . The suspension then was allowed to pass through a mesh sieve in to a beaker and the one left was discarded. After gentle shaking, the suspension was poured in to a conical centrifuge tube and was centrifuge at 1500 RPM for 3 minutes. After decanting the supernatant the sediment was agitated till thick homogenous fluid was obtained at the bottom of the tube and then was filled equal amount of water to the previous level. The content of the tube was mixed thoroughly with thumb over the open end of the tube. And then, using a pasture pipette, a 0.15 ml fluid was taken from the suspension and placed on a microscope slide covered with a cover slip. Then was examined under low power objective to check the presence of fasciola egg.
Data Management and Analysis
Data regarding coprological fecal examination, and associated risk factors were recorded on specially designed forms and entered to Microsoft Excel to undergone preliminary analysis. Descriptive statistics were computed. At a 95% confidence level, a Chi-square test was used to compare whether there was significant difference of prevalence of ovine fasciolosis between different risk factors such as sex, age body score, origin, months and different species group of animals. Prevalence was calculated as the number of parasitological positive animal examined divided by the total number of animal observed at that particular time (Michael, 2004).The result was analyzed by SPSS version 20 software.
Results
From a total of 500 faecal samples examined from small ruminant during the study periods, 305 samples were found positive for fasciolosis with an overall prevalence rate of 61% (Table 1) prevalence variation exists between the study woredas, the highest being at Haru woreda (71.19%) followed by mitikollo (61.64%), charosa (58.88%) and kedida (58.51%) at Kallu woreda (Table 3).
Table 1: Prevalence of small ruminant fasciolosis in caprine and ovine species
|
Animal Species |
Total examined (n) |
Coprological test |
Prevalence (%) |
|
|
Positive |
Negative |
|||
|
Caprine |
105 |
36 |
69 |
34.3 |
|
Ovine |
395 |
269 |
126 |
68.1 |
|
Total |
500 |
305 |
195 |
61 |
X2 cal = 39.8708, P = 0.000
Variation on the occurrence of ovine fasciolosis was computed among different variables such as sex, breed, body condition, age, species of small ruminant and seasonal occurrence. There was no statistical significant difference in the occurrence of ovine fasciolosis between male and female animals (P> 0.05) (Table 4).The Incidence of fasciolosis between local and cross breed animals was 61.7 and 56.9 percent respectively.
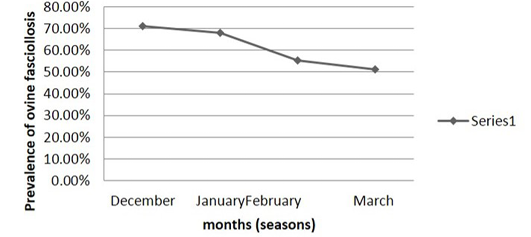
Table 2: Monthly (seasonal) prevalence of small ruminant fasciolosis in 3 kebeles of kallu woreda and harbu woreda of south wollo zone bordering borkena liver
|
Months (seasons) |
No. examined |
Coprological test |
Infectious rate |
|
|
Positive |
Negative |
|||
|
December |
125 |
89 |
36 |
71.2% |
|
January |
125 |
85 |
40 |
68% |
|
February |
125 |
69 |
56 |
55.2% |
|
March |
125 |
64 |
61 |
51.2% |
X2 cal = 10.44 98, P = 0.015
Infection rate between caprine and ovine species of animals were compared. The statistical analysis (34.29% and 68.10%) (Table 3) showed highly significant difference (P< 0.05). The monthly prevalence rates of small ruminant fasciolosis in the study area revealed high infection rates during the months of December to January that is during the dry season. The prevalence rates of fasciolosis decreases during the dry period from February to March (Table 2). On subjective basis evaluation of body condition of each sampled small ruminant were made in animals living under the same environmental conditions to see the effect of fasciolosis in debilitating (emaciating) infected animals. Infection rates of fasciolosis in “poor” body condition group was significantly (P< 0.05) higher than animals with good body condition group (Table 4).
Table 3: Comparison of prevalence among different study areas
|
Woreda (district) |
No. examined (n) |
Coprological test |
Prevalence (%) |
|
|
Positive |
Negative |
|||
|
Kedida |
188 |
110 |
78 |
59 |
|
Mitikollo |
146 |
90 |
56 |
61 |
|
Chorosa |
107 |
63 |
44 |
59 |
|
Harbu |
59 |
42 |
17 |
71.1 |
X2 = 3.2910, p-0.349
Table 4: Association of risk factors with prevalence of small ruminant fasciolosis
|
Variables |
No. examined(n) |
Coprological test |
Prevalence (%) |
p - value |
|
|
Positive |
Negative |
||||
|
Sex |
|||||
|
Male |
204 |
127 |
71 |
62.3 |
0.633 |
|
Female |
296 |
178 |
11 |
60.1 |
- |
|
Age |
|||||
|
Young |
125 |
40 |
85 |
320 |
0.000 |
|
Adult |
375 |
265 |
110 |
70.7 |
- |
|
Breed |
|||||
|
Local |
428 |
264 |
164 |
61.7 |
0.446 |
|
Cross |
72 |
41 |
31 |
56.9 |
- |
|
Body Condition |
|||||
|
Poor |
280 |
246 |
36 |
87.9 |
0.000 |
|
Good |
218 |
59 |
159 |
27.06 |
|
Discussion
Small ruminant fasciolosis exists in almost all regions of Ethiopia. However, the prevalence rate, epidemiology and fasciola species involved vary with locality. This is mainly attributed to the variation in the climatic and ecological conditions such as altitude, rain fall, and temperature and management systems of the small ruminants.
The present study was conducted in small ruminant for a period of five months in three woredas of South Wollo zone bordering Borkena River with an overall prevalence of 61%. The current prevalence was relatively higher than (Bogale et al., 2012) 45.6% in Oda Bultum district, Western Hararghe (Tesfaheywet, 2012), 49% in and around Dawa- Cheffa, Kemissei area (Bitew et al., 2010), 39.5% in Adigrat (Gebreizgabeher et al., 2012) but, spectacularly higher than the findings of Hawassa zuria (Rahmeto et al., 2014) 9.8%, 14.6% in Jimma area (Kedir et al., 2012), 14.6% in and around Hirna town (Mulatu et al., 2011). The maintenance of high prevalence of fasciollosis in the study area is strongly associated with the presence of favorable environments for the existence, multiplication and spread of intermediate host snails and the parasite (Fasciola). In most parts of the study areas, the feature of the land scape is slopy and swampy and water lodging areas were observed in the present study. Generally, the variation of this infection in these areas might be due to the variation in agro-ecological condition, geographical variation, number of study samples and climatic conditions of the areas (Ngategize et al., 1993).
Prevalence rate of 58.51, 61.64, 58.88 and 71.19 percent was recorded in kedida, mitikollo, chorose of kalu woredas and Harbu woreda, respectively. There was no statistically significant difference (P> 0.05) between the two woredas i.e. kallu woreda (kedida, mitikullo and chorosa) and Harbu woreda, this signified district seems have no impact on the infection rate and both woredas have the same on constant average humidity, average rainfall and average temperature. The reason was similar with Rahmeto et al. (2014) in Hawassa Zuria and Gebreyohannes et al. (2013) Menz Gera Midr Woreda of North Shoa Zone, Ethiopia.
Prevalence of 62.25% and 60.14% was recorded in male and female animals, respectively. There was no statistically significant difference (P> 0.05) between the two sexs. The result was similar with Mathewos et al. (2014), Rahmeto et al. (2014), Gebreyohannes et al. (2013) and Mulatu et al. (2011). This signifies sex seems have no impact on the infection rate and both male and females are equally susceptible and exposed to the disease.
In the present study the prevalence was 61.68% and 56.94% in local and cross breed of small ruminants, respectively. There was no stastically significant difference (P> 0.05) between the two breed, this signifies breed seems have no impact on the infection rate and both local and cross breed are equally susceptible and exposed to the disease. The result was in contrary to report by Desta et al. (2013), 60.3% in local and 73.9% in Cross breed. Experimentally, higher infection rate occurs in young animals (Soulsby, 1982; Radiostitis et al., 1994). Relatively lower infection rates observed in small ruminants of age group young (32%) compared to adult (70.67%) in line with previous studies of Mathewos et al. (2014), Rahmeto et al. (2014) and Gebreyohannes et al. (2013). It was suggested that to the fact that lambs are not often driven with older (adult) age groups to grazing and watering points.
Prevalence of fasciollosis in sheep was significantly higher than goats. Similar results were reported by Mulatu et al. (2011), Endris et al. (2008) and Michael et al. (2005) unlikely to the significant variation of the parasite, between sheep and goats (P > 0.05) as reported by Rahmeto et al. (2014). This is possibly because goats were selective grazer or browser that reduces opportunity of harboring the infective metacercaria of Fasciola which commonly encysted grass found around marshy areas.
The monthly (seasonal) variation in the prevalence of fasciolosis has been studied for 4 months (December – March) in the study area. In this study the highest prevalence rates were observed from December to January i.e. Infection rates of fasciolosis decrease in February during the dry season period of the year that predominates in this month. The lowest prevalence rate was observed in March. The seasonal pattern to Fasciola infection is related to the seasonal activity of the intermediate host snails. During the dry season (periods) breeding of the snails and development of the larval flukes slowdowns or stops completely and snails undergo a state of aestivation (Urquhart et al., 1994; FAO, 1994).
The results of the study indicated that the infection rates in poor body condition animals were significantly higher (P< 0.05) than that of the good body condition animals. The result was in agreement with Mathewos et al. (2014), Desta et al. (2013) and Mulatu et al. (2011). This signifies the importance of fasciolosis in causing weight loss and is the characteristic sign of the disease. Emaciation is the cause of production losses i.e. meat, milk and reproduce.
Conclusion
The present study conducted on small ruminant fasciolosis for a period of four month in two wordas i.e. kalu woredas (Kedida, Mitikolo and Cholosa) and Halbu woreda of south wollo zone bordering borkena river conclude that fasciolosis is the most wide spread and prevalent parasitic disease affecting the health and productivity of animals. Strategic anthelminthic treatment with appropriate flukicidal drugs should be practiced two times a year i.e. after the end of the dry season and after the end of the rainy season so as to eliminate the fluke burden of the host animals and minimize pasture contamination by reducing faecal egg out puts and thus interrupting the lifecycle of the parasite. Further study should be conducted on the epidemiology of the disease, biology and ecology of intermediate host snails (Lymnaea) to help avoid difficulties in planning and programming control strategies.
Author’s Contribution
Birhanu Tadesse completed his DVM degree by this paper and dedicatedly collected samples and wrote the article. Biniam Tadesse contributed in sampling, and laboratory analysis. Alemu Aylate was the co-advisor and helped in analysing the data, while, Ashenafi Kiros was the editor and advisor of the manuscript, by preparing the article in scientific manner and revising.
Acknowledgments
The author would like to thank Haramaya University for the fund to complete the research project and we also like to thank Kalu and Harbu Woreda Agricultural and Rural Development Office and Kombolcha Veterinary Diagnostic Laboratory staff for their unreserved support.
Conflict of interest
There exists no conflict of interest.
References