The Journal of Advances in Parasitology
Case Report
Human Asian Trypanosomiasis due to Trypanosoma evansi: A Rare Case
Vaishali Wabale1*, Prashant Nalage2, Ameeta Joshi1, Renu Bharadwaj1, Kalpana Deshpande3, Abhay Chowdhary1
1Department of Microbiology, Grant Government Medical College, Mumbai; 2Preclinical Toxicology, Suven Life, Science, Hyderabad, Telangana; 3Department of Pathology, Grant Government Medical College, Mumbai, India.
Abstract | Trypanosomes are unicellular eukaryotic haemoprotozoan parasite found worldwide. They infect humans, domestic and wild animals and are transmitted by blood-sucking insects. Here, the patient, a pregnant female, in the 28th week, a known case of retroviral disease (RVD) presented with severe anemia and upper respiratory tract infection (URTI) with tuberculous lymphadenitis. Preliminary diagnosis was made was threatened abortion with respiratory tract infection due to complaints of breathlessness on exertion, cough with expectoration, low grade fever since one month along with pain in abdomen and in ear since 15 days. The direct microscopic examination of Leishman stained PBS smear of blood revealed a flagellated form with a purple colored nucleus with a dark red kinetoplast in blue colored cytoplasm and an undulating membrane. A provisional diagnosis of Trypanosomiasis was made and confirmed when T. evansi grew on Novy- Mac Neal- Nicolle (NNN) Modified Twin pack Medium (HI MEDIA Laboratories, Mumbai). The patient responded to suramin. Though human serum is lethal to Trypanosma evansi, but because the patient was immunocompromised, hence this species led to disease with teaming parasitaemia.
Keywords | Human Asian Trypanosomiasis, Trypanosoma evansi, Retroviral disease, Suramin.
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | July 23, 2015; Revised | September 13, 2015; Accepted | September 16, 2015; Published | November 22, 2015
*Correspondence | Vaishali Wabale, Grant Government Medical College & Sir J. J. Hospitals, Byculla, Mumbai, Maharashtra, India; Email: vrwabale@gmail.com
Citation | Wabale V, Nalage P, Joshi A, Bharadwaj R, Deshpande K, Chowdhary A (2015). Human Asian trypanosomiasis due to Trypanosoma evansi: A rare case. J. Adv. Parasitol. 2(3): 65-68.
DOI | http://dx.doi.org/10.14737/journal.jap/2015/2.3.65.68
ISSN | 2311-4096
Copyright © 2015 Wabale et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Trypanosomiasis is a mechanically transmitted vector borne haemoprotozoan disease, caused by the genus ‘Trypanosoma’. It is widely distributed in animals and human beings and is usually found in domestic and wild animals like cattle and horses (Joshi, 2013; Surra, 2009; Gutierrez et al., 2010). In India, the disease known as “Surra” and is caused by Trypanosoma evansi, is transmitted through a non-cyclical method by biting flies, such as ‘Tabanidae’. In Africa, another but related species T. brucei is transmitted cyclically by a different species of tsetse fly (Glossina spp.) (Limaye and Ganvir, 2015). T. evansi is thought to be derived from T. brucei by deletion of maxi circle kinetoplastic deoxyribonucleic acid (DNA) material that is required for cyclical development in tsetse flies (Desquesnes et al., 2013). In tropical country like India, tsetse fly vector is absent (Joshi, 2013). The Office International Epizooties (OIE) mentions this disease under list B diseases of significance in horses (Limaye and Ganvir, 2015). However, until now there is no vaccine available for Surra (OIE Terrestrial Manual (2012).
In 1881, Griffith Evans, an English veterinary surgeon identified T. evansi in horses and camels from the Punjab region of India (Joshi, 2013). It is thought to produce immunosuppression resulting in concurrent infection and poor immune response to vaccines (Gupta et al., 2009; Singla et al., 2009). Though T. evansi is not known to have a zoonotic potential, it is the most widely distributed species in India and Asia (OIE Terrestrial Manual (2012). It normally found in animals causing an acute disease in camels and horses as well as a chronic disease in cattle and buffaloes (Juyal, 1998; T. evansi Wikipedia).
The increasing number of human cases being reported may herald an era when we will observe that the parasite is now developing the ability to spread to humans and cause disease (Gupta et al., 2009). If this occurs, it would result in a wider distribution of T. evansi in humans even more than the commonly occurring T. brucei (Brun , 2005).
Here we present a rare case of Human Asian Trypanosomiasis due to T. evansi in a retroviral disease (RVD) patient with lymphadenitis from Mumbai, Maharashtra.
A 23 year old married female: G3P2L2 currently pregnant, in the 28th week, presented with severe anemia and upper respiratory tract infection (URTI) to the Sir JJ Group of Hospitals Mumbai. She was a known case of RVD. She came with complaints of breathlessness on exertion, cough with expectoration and low grade fever since one month. She also complained of pain in abdomen and pain in ear since 15 days. Preliminary diagnosis was made was threatened abortion with Respiratory tract infection. She had a past history of pulmonary tuberculosis (TB) with cervical lymphadenitis. She had been put on Anti Koch’s Treatment (AKT) which she stopped on her own after 3 months.
On examination pedal edema was present. Pulse was 110/m, BP 120/70 mm Hg and RR 30/min. A marked hepatosplenomegaly (16.5 cm) and splenomegaly (12.9 cm) was detected. Peripheral blood smear showed hypochromia, anisocytes, polychromosis, macrocytes, poikilocytes and basophilic stippling. Platelet count was 1, 15,000/cumm. Solubility test for sickle cell was negative. Patient was diagnosed as a case of dimorphic anemia. Hemoglobin was below 4 gm%, RBC 100 mg%, Urea 24 mg%, Random sugar 100 mg%, Na 131 mg/lit and K 4.9 mg/lit. WBC were 9.33 X 109/lit and PLT were 253 X 103/lit.
The left cervical lymph node on biopsy was showing a picture consistent with tubercular lymphadenitis. Bone marrow trephine biopsy showed granulomatous inflammation suggestive of TB. Fine needle aspiration cytology (FNAC) smear examination by Ziehl-Neelsen staining (ZN) showed presence of acid fast bacilli (AFB). Serological investigations revealed negative result for Dengue, Leptospira, Veneral Disease Research Laboratory (VDRL) test as well as HBsAg and CD3+CD4 ratio was 74%.
Patient came to know her seropositive status for Human immunodeficiency virus (HIV)-1 during her first pregnancy in Mumbai. Husband contracted HIV through heterosexual route from a prostitute during his visit to Africa before marriage. A blood smear examination showed the presence of unknown flagellates, hence referred for further parasitological confirmation.
In the microbiology department, a direct microscopic examination of thick and thin peripheral blood smear (PBS) was performed, and stained with Leishman stain. Thick smear revealed numerous parasites with typical morphology of purple colored nucleus, dark red kinetoplast and flagellum in blue colored cytoplasm with undulating membrane, confirming the presence of trypanosome as shown in Figure 1.
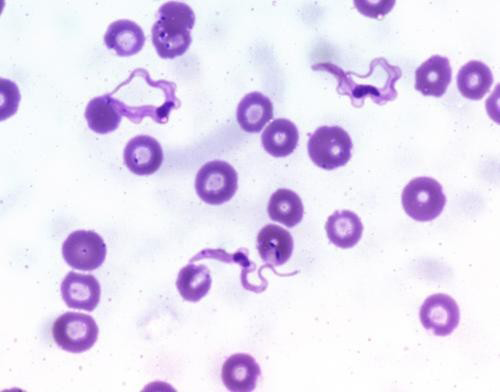
Figure 1: Thin PBS smear showing centrally located nucleus with a sub terminal kinetoplast at sharp posterior end and large undulating membrane with free flagellum (Leishman stain, 1000X)
Bone marrow trephius biopsy smears stained with Leishman stain revealed trypanosome forms. The FNAC also showed numerous flagellated trypomastigote forms. Three consecutive blood samples were collected within an interval of 72 hours by venipuncture using heparinized vacutainer (Becton Dickinson) tubes and processed for parasitological culture. All these samples were plated on freshly prepared Novy- Mac Neal- Nicolle (NNN) Modified Twin pack Medium (HI MEDIA Laboratories, Mumbai). T. evansi
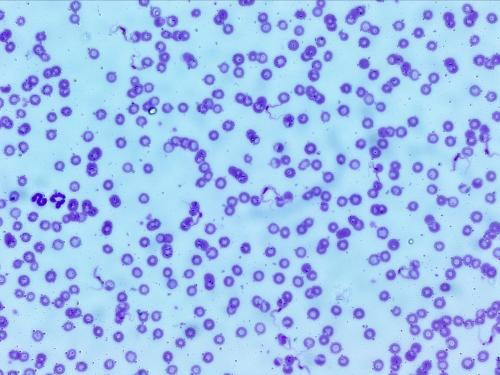
Figure 2: Thin smear of the culture on NNN media showing plenty of long, slender, flagellated, (15-34 µm) dividing forms of trypanosomes (Leishman stain, 1000X)
grew on this media. The thin smears prepared from positive culture stained with Leishman stain showed monomorphic, slender, flagellated, dividing trypomastigote forms of trypanosomes (6 x 106 trypanosomes/mL) as shown in Figure 2 and binary division stage in Figure 3.
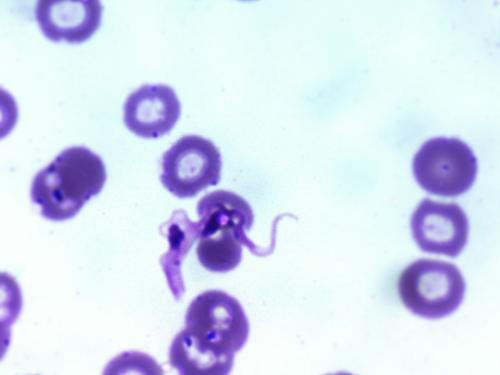
Figure 3: Binary division stage of trypanosome
Hence by standard parasitological screening and culture techniques, T. evansi was isolated and identified.
The patient was treated with suramin solution at the dose of 1 gm intravenous infusion weekly. Total 5 injections were administered. Follow-up: Blood as well as cerebrospinal fluid (CSF) were serially examined to look for the presence of parasites for another 6 to 12 months. Post treatment, the parasitic load decreased. Stout, stumpy forms were still present in the blood and persisted up to 2 months. Parasites could not be detected in the blood in the 3, 6 and 12 months post treatment visits. Patient did not show any side effects, fever or any major complaint during the course of treatment to keep a vigilance on relapse. In addition, the overall patient’s general state of health was improved.
Trypanosomes cause various diseases in animals as well as in humans. Among them, T. evansi has a wider geographical distribution and was first reported in the year 1885. This species is present in Africa, Asia, South America and in Middle East. It infects a wide range of animals like horses, cattle’s, buffalos, deer’s, camels and mules (Brun, 2005). Virulent nature of the infecting strain and immune make-up of the affecting host, plays an important role in the pathogenicity (Brun, 2005). Humans are naturally protected against most of the animal pathogenic trypanosomes because of their sensitivity to the lytic factor present in the human plasma (Brun, 2005). Though human plasma is lethal to this organism, in the present case, the patient was immunocompromised and this may have facilitated the infection.
Total nineteen cases of ‘atypical human trypanosomes’ (a-HT) have been reported worldwide. From these, 5 were of T. evansi (Joshi, 2013; Singla et al., 2009). The overall environmental conditions in India are favorable for the parasite and facilitate zoonotic transmission (Joshi, 2013). It species has emerged as a potentially pathogenic species for humans in the recent years. Over a period of 37 years (year 1974 - 2010) a total 15 human cases have been recorded; out of these nine cases were reported after 2003. Few cases were identified by microscopy and remaining with the help of molecular tools (Singla et al., 2009).
The first case of human infection due to T. evansi from the central part of India (Seoni village, Sindevahi taluka, Chandrapur district, Maharashtra state) was reported by Joshi (2005). The patient was a cattle farmer who contracted this disease from cattle. Entry was probably through a wound. The Director of General Health Services (DGHS) of Maharashtra conducted a serologic survey in the local population in Seoni village with the assistance of WHO and the Institute de Recherche pour le Development (France) (Shegokar et al., 2006). The agenda for investigation was looking for microscopic occurrence of possible appearance of T. evansi trypanosomes in the blood and serologic screening, especially exposed population associated with the cows, by using card agglutination test for trypanosomiasis (CATT), which was CATT/T. evansi, (Institute of Tropical Medicine (ITMA) Antwerp, Belgium ] (Pathak et al., 1997). The authors found that the serologic results were proportional to parasitemia in the human exposure to T. evansi (Shegokar et al., 2006).
The negative results of the survey could have been due to the fact that humans are usually resistant to T. evansi because of trypanolytic factor present in human serum which is an apolipoprotein L1 (Gupta et al., 2009; Shegokar et al., 2006). Hence, though T. evansi is not infective to humans, the follow-up studies of seropositive persons are required to observe the evolution of human infection with this parasite. There is also a necessity to monitor whether febrile episodes occur in any of such patients (Shegokar et al., 2006). With the description of this new emerging zoonosis, Reto Brun christened it as ‘Human Asian Trypanosomiasis’ which has a potential to cause an imminent threat to human health (Brun, 2005).
In the present case, T. evansi was isolated from a HIV positive pregnant patient. Here, the patient responded to suramin. An early diagnosis resulted in appropriate therapy with suramin resulting in a cure. Appearance of so many a-HT cases in recent years could be an indication of an emerging new zoonosis or may represent an unlucky biological accident and is a matter of concern (Joshi, 2013). The documented reports from India may be representing the tip of the iceberg, and many more undetected as well as unreported cases might be present. Improvement in available diagnostic techniques, particularly molecular assays can make diagnosis easier. Lack of awareness and no access to health care services may increase the spread of infections in humans. However it is expected that as long as T. evansi strains do not invade the central nervous system and remain sensitive to standard drugs such as suramin the new form of human trypanosomiasis will probably not develop into major health problem (Brun, 2005).
To conclude, the need of the hour is to have a system in place to tackle ‘Human Asian Trypanosomiasis’, an emerging new zoonosis. Policies should be made to investigate disease prevalence and arrangement for accessible accredited laboratories for thorough parasitological examination. Utmost care should be taken while handling the livestock to reduce the accidental human infections.
Acknowledgment
We thank Dr. Mukulesh L. Gatne (Professor and University Head, Department of Parasitology, Bombay Veterinary College, Parel, Mumbai, 400 012, Maharashtra).
Conflict of interest
There exixits no conflict of interest.
AUTHORS CONTRIBUTION
Vaishali Wabale acted as principal investigator and presented concept and design of the study, Prashant Nalage assisted in all microbiological investigations and eterinary investigations while Ameeta Joshi assisted in writing an article. Renu Bharadwaj assisted in culture and confirmation of parasite; Kalpana Deshpande assisted in thin and thick smear examination and Abhay Chowdhary reviewed and edited the article.
References





