The Journal of Advances in Parasitology
Research Article
Community Structure Analysis Metazoan Parasites of Channa punctatus (Bloch, 1800) from Meghadrigedda Reservoir of Visakhapatnam District, Andhra Pradesh, India
Anuprasanna Vankara1*, Vijayalakshmi Chikkam2
1Department of Animal Sciences, Yogi Vemana University, YSR Kadapa, A.P, 516 003, India; 2Department of Zoology, Andhra University, Visakhapatnam, 530 003, India.
Abstract | One hundred eighty two Channa punctatus collected from various freshwater bodies of Visakhapatnam District during 2007-2009 were examined for the presence of metazoan parasites of which 128 (70.3%) fish were parasitized by at least one or more metazoan parasite species. A total of 7 metazoan parasites were obtained during the present investigation of which 5 are endoparasites i.e. 3 Digeneans- Genarchopsis goppo Ozaki, 1925, Allocreadium handiai Pande, 1937, Metacercaria of Euclinostomum heterostomum (Rud, 1809) Travassos, 1928, 1 cestode- Senga viskhapatnamensis Rama Devi and Rao, 1974 and 1 acanthocephalan- Pallisentis ophiocephali (Thapar, 1930) Bayliss, 1933; while only 2 were ectoparasitic copepods- Lernaea bengalensis Gnanamuthu, 1951 and Lamproglena chinensis Yu, 1937. Endoparasites predominate the majority of the components of the infracommunities analysed and represented 90.5% of the total parasites collected. The Shannon weiner index (H’) and evenness (E) were applied to the fully sampled metazoan infracommunities. The digenean, Genarchopsis goppo was most prevalent in the parasite community of C. punctatus but occupies the position of satellite species. The acanthocephalan, Pallisentis ophiocephali is the second prevalent species and occupies the position of the secondary species. There are no core species in the community. An insignificant positive correlation (r=0.012) was observed between the standard length of host and parasitic abundance of all parasites in the sampled fishes. Only two species Pallisentis ophiocephali and Genarchopsis goppo showed over-dispersed distribution pattern while the rest of the other species showed random distribution pattern. Host sex has no influence on overall parasitic abundance (Z=-1.65, p=0.05). Jaccard’s index was employed to observe the interspecific assosciation between each pair of parasite species within a same host. The higher JI values indicate there is very less competition among species as occupy different niches within the same host.
Keywords | Parasite ecology, Community structure, Channa punctatus, Visakhapatnam
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | June 22, 2015; Revised | August 18, 2015; Accepted | August 20, 2015; Published | October 02, 2015
*Correspondence | Anuprasanna Vankara, Yogi Vemana University, YSR Kadapa, India; Email: dr.anu@yogivemanauniversity.ac.in
Citation | Vankara A, Chikkam V (2015). Community structure analysis metazoan parasites of Channa punctatus (Bloch, 1800) from Meghadrigedda Reservoir of Visakhapatnam District, Andhra Pradesh, India. J. Adv. Parasitol. 2(3): 57-64.
DOI | http://dx.doi.org/10.14737/journal.jap/2015/2.3.57.64
ISSN | 2311-4096
Copyright © 2015 Vankara and Chikkam. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Snake head Murrel, Channa punctatus Bloch, 1800 is the representative of the family Channidae and forms an important inland fishery in Indian subcontinent. Channa punctatus, commonly known as Matta gorasulu, is one of the popular commercial fish highly relished in Southern India as delicious proteinaceous food. It is mud-loving fish due to its food habit which enables to serve as an intermediate host for many helminth parasites. Parasites occupy an imperative position in animal kingdom for their widespread adoption and detrimental activities to host (Hoffman, 1967). Every parasite residing in or on a fish inflict some degree of harmful effect on its host. Heavily infected fish shows an episodic or inhibited growth. The parasite community composition in a fish depends upon many environmental factors such as location of the habitat, season of the year; physio-chemical factors of water and fauna present in and around the habit and these factors can contribute to the emergence of new species and may increase the parasite species richness (Pavanelli et al., 1997). Also some factors such as diet, age, abundance of fish length, sex and interdependence of members of parasite fauna within fish and season directly influence parasitic fauna of the host (Ricklef and Schluter, 1993; Machado et al., 1994; Malhotra and Benarjee, 1997; Lizama et al., 2006; Puinyabati et al., 2013; Ravi and Yahaya, 2015). The parasitic interaction in various trophic levels, food webs, competition and biodiversity around them helps to shape their community structure. Many workers have worked on systematics of parasites, biology of fish, ecology of parasites and histopathology of tissues infected by parasites (Ramadevi and Rao, 1974; Kanth and Srivastava, 1987; Koul et al., 1991; Chowdhary, 1992; Sinha et al., 2008; Wani and Magray, 2008; Pardeshi and Hiware, 2011; Chandra et al., 2011; Gupta et al., 2012; Verma and Capoor, 2013). However, very few investigations on parasite community structure of freshwater fishes was conducted worldwide by Sirikanchana (1983), Nahar et al. (1993), Parween and Rahman (2000), Poulin (2001), Chaiyapo et al. (2007), Chandra (2008), Alam et al. (2010), Ghani and Bhuiyan (2011), Rakibuzzaman et al. (2011), Ayaz et al. (2013), Miah et al. (2013) and Bhuiyan et al., (2014). These types of ecological studies are scarce in Indian Sub-continent especially in Andhra Pradesh. An attempt was made to determine the parasite diversity of C. punctatus at component and infra-community structural levels and to evaluate the possible influence of host standard length and sex on its parasitic abundance.
Study Area
Meghadrigedda, a non-perennial drainage system is one of the prominent river catchments originated in hilly terrain of Visakhapatnam and Vizianagaram Districts of Andhra Pradesh extended in an area of 368Km2 including the reservoir area and located at 17˚42ʺ to 17˚57ʺ northern latitude and 83˚0ʺ to 83˚17ʺ eastern longitude (Figure 1). It is one of the major water resources of Visakhapatnam District enriched with a wide variety of fish fauna infected with varied parasites.
MATERIAL AND METHODS
The present study was designed to investigate the parasites of the snake headed murrel, channa punctatus collected from the Meghadrigedda reservoir of Visakhapatnam District, Andhra Pradesh from local fish markets during 2007-2009. A total of 182 C. punctatus were transported alive to the laboratory in aerated bags and were screened for the presence of parasites. Length, weight and sex each fish was noted carefully and all the organs were examined separately under the stereozoom microscope. Conventional techniques were employed to prepare permanent slides of the collected parasites (Hiware et al., 2003; Madhavi et al., 2007).
Calculation and Data Analysis
Structure of the component and infra communities of C. punctatus were described following the terminologies of Margolis et al., (1982) and Bush et al. (1997). Parasite infracommunities were described in terms of prevalence, abundance and intensity however component communities were described in terms of species richness, mean abundance, mean intensity and community similarity such as dominance, diversity and evenness indices. Species were classified according to Bush and Holmes (1986) as central/core species (if prevalence >66.6%), Secondary species (prevalence from 33.3% to 66.6%) and satellite species (prevalence <33.3%) of total number of fish analysed. Dispersion index (DI) was used to evaluate the dispersion pattern of parasite species. The distribution of parasites were classified as aggregated (DI>1.96), regular (DI<1.96) and random (DI < 1.96). Parasite diversity of the sample was calculated using Simpson (1949) diversity index (λ) and for infinite population, Shannon’s (Shannon and Weaver, 1949) index of diversity (H’) was used. For evenness, Shannon-based evenness was calculated. Host Size is one of the decisive factors in determining the parasitic communities in any host. Pearson linear correlation coefficient (r) was computed to determine possible correlation between host standard length with prevalence and abundance of each parasite species respectively (Zar, 1996). The existence of association between species for measuring degree of association is done by Jaccard’s Index (JI) whose value ranges between 0-1 and as the value approaches to 1, indicates the association between species is high. Mann-whitney U-test was used as an indication to scrutinize the influence of host sex on the parasitic abundance (Vassarstat.net/utest.html). Community
Table 1: Diversity parameters and distribution patterns of parasitic species of Channa punctatus (n=182)
|
Name of the parasite |
Infected fishes |
No. of parasites |
Prevalence |
Mean intensity |
Mean abundance |
D.I |
Location |
Nature of infection* |
Nature of species ** |
|
P.ophiocephali |
67 |
133 |
36.8 |
1.99 |
0.73 |
0.30 |
Intestine |
common |
Secondary |
|
G.goppo |
54 |
188 |
29.7 |
3.48 |
1.03 |
0.42 |
Stomach, Intestine |
frequent |
Satellite |
|
A.handiai |
34 |
65 |
18.7 |
1.91 |
0.36 |
0.15 |
Intestine |
frequent |
Satellite |
|
E.heterostomum |
5 |
7 |
2.7 |
1.40 |
0.04 |
0.02 |
Oesophagus |
sporadic |
Satellite |
|
S.visakhapatn amensis |
8 |
12 |
4.4 |
1.50 |
0.07 |
0.03 |
Intestine |
rare |
Satellite |
|
L.bengalensis |
25 |
38 |
13.7 |
1.52 |
0.21 |
0.09 |
Skin |
frquent |
Satellite |
|
L.chinensis |
4 |
4 |
2.2 |
1.00 |
0.02 |
0.01 |
Gills |
sporadic |
Satellite |
*Common= 30-50%; Frequent= 10-30%; Rare= 4-10%; Sporadic= <4%; **Core sp. =>66%; Secondary sp. =between 66-33%; Satellite sp. = <33%
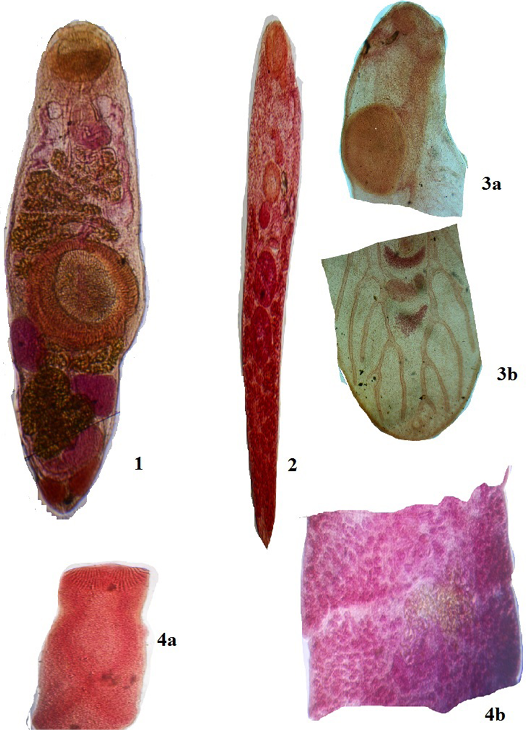
Figure 2: Plate 1
1: Genarchopsis goppo Ozaki, 1925, 4 х 10X; 2: Allocreadium handiai Pande, 1937, 4х10X; 3a: Metacercaria- Euclinostomum heterostomum (Rud, 1809) Travassos, 1928 Anterior region 4 х 10X; 3b: Metacercaria- Euclinostomum heterostomum (Rud, 1809) Travassos, 1928 Posterior region 4 х10X; 4a: Senga Visakhapatnamensis Rama Devi & Rao, 1974- Scolex, 4 х 10X; 4b: Mature proglottid, 4 х 10X
structure of parasites has been determined as a function of host habitats, sizes and sexes. All the statistical tests were conducted using excel in MS-Office and statistical significance level adopted was p≤ 0.05.
RESULTS AND DISCUSSION
Component Community Structure
Seven metazoan parasites were recorded during the present study of which 3 were digeneans identified as Genarchopsis goppo, Allocreadium handiai and Euclinostomum heterostomum; 1 cestode, Senga visakhapatnamensis; 1 Acanthocephalan, Pallisentis Ophiocephali and 2 copepods, Lerneae bengalensis and Lamproglena chinensis (Table 1, Figure 2 and 3).
Digeneans are most abundant of the parasite community (58%) of total parasites collected (Table 2). Of the 182
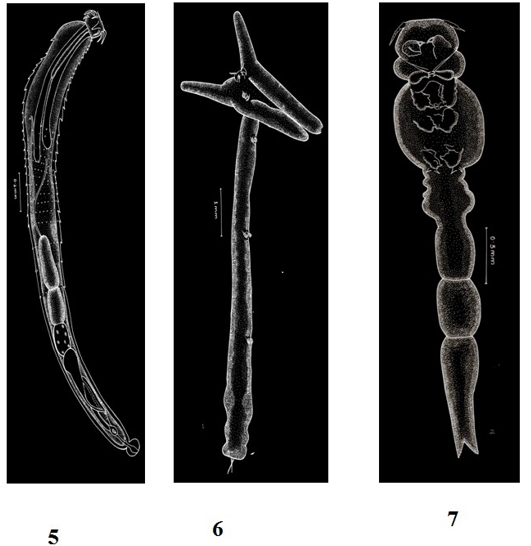
Figure 3: Plate 2
5: Pallisentis ophiocephali (Thapar, 1930) Bayliss, 1933-Line diagram; 6: Lernaea bengalensis Gnanamuthu, 1951- Line diagram; 7: Lamproglena chinensis Yu, 1937- Line diagram
hosts, 75 hosts (41.2%) of C. punctatus showed infection with any single parasitic group, 44 hosts (24.17%) showed infected with any two parasitic groups, 8 hosts (4.39%) showed infection with 3 parasitic groups and only 01 host (0.54%) showed infection with all the 4 parasitic groups (Table 3). Investigation on infestation with endohelminth and ectoparasites shows that infestation with endohelminth community (90.6%) was more than ecoparasitic community. Among the 3 digeneans, E. heterostomum was metacercarial larval form whereas the other two are adults i.e G. goppo which is the most dominant species with 188 parasites (Range=1-15) collected (42% of total parasites) with a mean of 1 parasite/fish and showed a highest values of mean relative dominance of 0.42 (Table 1) and Allocreadium handiai which is the third dominant species with 65 parasites (Range=1-3) collected.
Table 2: Frequency distribution of number of parasitic groups per individual in C. punctatus
|
S. No. |
Parasite group |
No. of parasites |
Dominanceindex |
Mean total parasites |
|
1 |
Digeneans |
260 |
0.58 |
1.42 |
|
2 |
Cestodes |
12 |
0.02 |
0.065 |
|
3 |
Acanthocephalans |
133 |
0.29 |
0.73 |
|
4 |
Copepods |
42 |
0.09 |
0.23 |
n = 182; Σx = 4; X = 4/182= 0.022; Range = 1-4
Table 3: Number of parasites obtained, dominance index and mean total parasites of different parasitic groups in C. punctatus
|
S. No. |
No. of parasitic groups |
No. of infected fishes |
% of frequency |
|
1 |
1 |
75 |
41.2 |
|
2 |
2 |
44 |
24.17 |
|
3 |
3 |
8 |
4.39 |
|
4 |
4 |
1 |
0.54 |
Table 4: Frequency distribution of number of parasitic species per individual in C. punctatus
|
S. No. |
No. of parasitic species |
No. of infected fishes |
% of frequency |
|
1 |
1 |
70 |
38.46 |
|
2 |
2 |
49 |
26.92 |
|
3 |
3 |
7 |
3.84 |
|
4 |
4 |
2 |
1.09 |
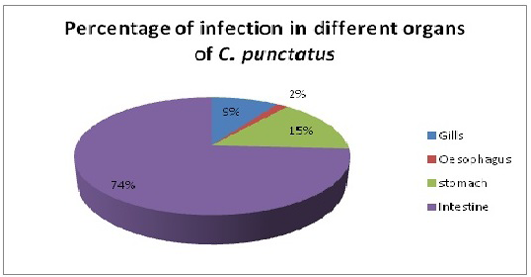
Only single species of Acanthocephalan, P. ophiocephali (29.7%) all parasites collected occupies the second dominant position in the parasite assemblages after G. goppo. Lernaea bengalensis collected from the skin of the host was the fourth dominant species of the community with 38 parasites (20.8%) of all the parasites collected. However, the other 3 species digenean, E. heterostomum (only 7), cestode, S. visakhapatnamensis (only 12) and copepod, L. chinensis (only 4) were sporadic and rare in occurrence. Alimentary canal especially oesophagus, stomach and intestine being the most preferred spots of the endoparasites whereas gills and skin being much preferred habitats of ectoparasites and these parasites maintain a commensalistic relationship with its host and do not create any menace though present in abundant number (Reichenbach-klinke and Elken, 1965; Begum and Banu, 2012). The number of parasites in gills, oesophagus, stomach and intestine of C. punctatus were screened for the presence of parasites. Intestine showed the maximum infection (74%) with the endoparasites followed by stomach (14.9%) and gills (9.39%) (Figure 4). The present study correlates with the views of Adebanjo (1979), Smith (1981) and Das and Goswami (2014) who suggested that the occurrence of more parasites in the intestine than in stomach might be due to the presence of digested food in the intestine or due to greater surface area of the intestine. Larvae actually survive in the intermediate hosts by encapsulating themselves in tough sheath inside the body cavity and manage to reach the final hosts i.e birds to complete their life-cycle (Barson, 2004). In the present survey, only one larvae E. heterostomum was found encapsulated in a tough sheaths inside the body cavity (oesophageal cavity) which will reach their final hosts (herons). The prevalence and intensity of the parasite community might be influenced by age, length of host, changes in diet, in the volume of food ingested, changes in immunological competence and changes in the probability of contact with intermediate hosts.
Infra Communities
A total of 128 (70.3%) fishes were parasitized by at least one or more parasite species. A sum of 447 individual parasites was collected with a mean of 2 parasites. Seventy hosts (38.46%) showed infection with one parasite species and 49 (26.9%), 7 (3.84%) and 2 (1.09%) showed multiple infections with 2, 3 and 4 parasite species respectively (Table 4). The lower values of Shannon’s Hʹ index (0.62) and Simpson index (0.47) specifies less diversification of
Table 5: Diversity parameters of metazoan parasite communities of C. punctatus
|
Host |
Sample size |
Mean no. of parasite species |
Simpson’s diversity index |
Shannon’s diversity index (H') |
Shannon-based evenness (E) |
No. of core species |
|
C. punctatus |
182 (128) |
2 |
0.47 |
0.62 |
0.868 |
- |
Table 6: Mean (X), Variance (s2) and Dispersion index (s2/X) of parasite species in C.punctatus
|
Name of the parasite |
2005-2007 (n =182) |
|||
|
No. of parasites collected |
Mean (X) |
Variance (s2) |
Dispersion index (s2/X) |
|
|
Pallisentis ophiocephali |
133 |
0.73 |
1.78 |
2.45 |
|
Genarchopsis goppo |
188 |
1.03 |
5.01 |
4.86 |
|
Allocreadium handiai |
65 |
0.35 |
0.65 |
1.85 |
|
E.heterostomum |
7 |
0.03 |
0.059 |
1.97 |
|
S.visakhapatnamensis |
12 |
0.065 |
0.106 |
1.63 |
|
L. bengalensis |
38 |
0.208 |
0.33 |
1.59 |
|
L. chinensis |
4 |
0.02 |
0.02 |
1.08 |
parasitic community. The slightly higher value of Shannon based evenness (0.868) suggests that community structures show consistent distribution of all parasite species (Table 5). Only P. ophiocephali was commonly occurring parasite with a prevalence ranging from 30-50% in the hosts while the 3 species, G. goppo, A. handiai and L. bengalensis are frequent in occurrence with a prevalence ranging between 10-30%. S. visakhapatnamensis is rare in occurrence (4-10%) and rest of the 2 species E. heterostomum and L. chinensis are sporadic in occurrence (<4%). There is no core species in the host. Pallisentis ophiocephali is the only one secondary species while the remaining species occupies the position of satellite species (Table 1). Berger-Parkers dominance index was calculated for each species of parasite in the host. Genarchopsis goppo represented the highest dominance value followed by P. ophiocephali and A. handiai respectively (Table 1). The ratio of variance to mean values gives the index of dispersion (DI). Pallisentis ophiocephali and G. goppo exhibits over-dispersed (aggregated) distribution in host body while the other species show their random distribution (Table 6). Poulin (1993) opined that aggregated distribution of the parasite population is one of the common features of metazoan parasite infection. The present study supports the views of Anderson and Gordon (1982) who suggested that aggregated pattern might be due varied behavioral changes of the host, susceptibility and capability of host immunological response.
Infection with Respect to Host Standard Length
Many scientists were of the opinion that the relationship between host length and parasite abundance might be due to the occurrence and relationship between parasite diversity and body length of sample (Jha and Sinha, 1990; Saad-Fares and Comb, 1992; Zelmer and Arai, 1998; Poulin, 2000; Lizama et al., 2005, 2006; Singhal et al., 2009; Koyun, 2012; Kaur et al., 2013). According to Pearson’s correlation coefficient, there exists a very insignificant positive correlation (r= 0.012) between host length and parasitic abundance of C. punctatus (Table 7). Younger age fishes are more susceptible to parasite infection than the older ones. The present study is in accordance with the views of Lewerts (1958) and Dobson (1962) who reported that the penetration of parasite larvae is easier in younger fishes than older ones.
Table 7: Parasitic abundance in the different size classes of C. punctatus
|
S. no. |
Groups |
Size |
parasites |
Correlation coefficient, R |
|
1 |
Group-1 |
10-20 cm |
344 |
|
|
2 |
Group-2 |
20-30 cm |
97 |
0.011506 |
|
3 |
Group-3 |
30-40 cm |
0 |
|
|
4 |
Group-4 |
40-50 cm |
6 |
Infection in Relation Host Sex
Reports on the prevalence of parasites with respect to host sex are varied. There are few reports which suggest that male hosts showed more infection than females (Zelmer and Arai, 1998; Takemoto and Pavanelli, 2000) while some were of the opinion that females are more infected than males (Ibiwoye et al., 2004; Maan et al., 2006; Singhal and Gupta, 2009). The present study holds true with the views of Jarkovsky et al. (2004) who suggested that there are no significant differences in infection rate of male and female hosts. In the present survey of 182 fishes, 103 were males and 79 were females of which 71(68.9%) male and 57 (72.5%) female fishes were infected with at least one parasite. According to Mann-Whitney Z (U) test, there is no significant relation between host sex and parasite abun
Table 8: Diversity parameters of parasitic species in males and females and values of Mann-Whitney U-test to evaluate rate of host sex and parasitic abundance in C. punctatus
|
Host name |
Channa punctatus (Nm = 103; Nf = 79) |
Mann –Whitney U test (Z) |
|||||||||
|
Parasite |
Nmi |
Pm |
MIm |
MAm |
Nfi |
Pf |
MIf |
MAf |
Z (U) |
P1 (significance level) |
P2 (significance level) |
|
P. ophiocephali |
31 |
30.09 |
1.61 |
0.485 |
36 |
45.5 |
2.30 |
1.05 |
2.31 |
0.0104 |
0.0209 |
|
G. goppo |
31 |
30.09 |
3.91 |
0.96 |
23 |
29.1 |
3.86 |
1.12 |
-1.26 |
0.1038 |
0.2077 |
|
A. handiai |
27 |
26.21 |
1.96 |
0.51 |
7 |
8.86 |
1.71 |
0.15 |
0.68 |
0.248 |
0.496 |
|
E. heterostomum |
2 |
1.94 |
1 |
0.019 |
3 |
3.79 |
1.66 |
0.03 |
- |
- |
- |
|
S. visakhapatnamensis |
3 |
2.91 |
1.33 |
0.038 |
5 |
6.32 |
1.6 |
0.10 |
- |
- |
- |
|
L. bengalensis |
12 |
11.65 |
1.08 |
0.126 |
13 |
16.4 |
1.92 |
0.31 |
-2.94 |
0.0016 |
0.033 |
|
L. chinensis |
4 |
3.88 |
1 |
0.038 |
0 |
0 |
0 |
0 |
- |
- |
|
*Nm = Number of males examined; Nf = Number of females examined; Nmi = Number of males infected; Nfi = Number of females infected; Pm& Pf = Prevalence of males and females respectively; MIm&MIf = Mean intensity of males and females; MAm & MAf= mean abundance of males and females, respectively.
Table 9: Values of Jaccard’s Index (JI) to estimate interspecific association between each pair of parasite species of C.punctatus
|
Parasites |
P. ophiocehali |
G. goppo |
A. handiai |
E. heterostomum |
S. lucknowensis |
L. chinensis |
L. bengalensis |
|
P. ophiocehali |
- |
0.492 |
0.253 |
0.029 |
0.114 |
0.029 |
0.047 |
|
G. goppo |
0.492 |
- |
0.078 |
No association |
0.053 |
0.037 |
0.026 |
|
A.handiai |
0.253 |
0.078 |
-- |
No association |
0.052 |
0.058 |
0.036 |
|
E. heterostomum |
0.029 |
No association |
No association |
- |
No association |
No association |
No association |
|
S. lucknowensis |
0.114 |
0.053 |
0.052 |
No association |
- |
0.1 |
No association |
|
L. chinensis |
0.029 |
0.037 |
0.058 |
No association |
0.1 |
- |
No association |
|
L. bengalensis |
0.047 |
0.026 |
0.036 |
No association |
No association |
No association |
- |
dance (Z=1.65, P=0.05) (Table 8). The insignificant values with respect to sex from Z (U)-test shows that the ecological relationship of both males and females might be similar. However, individual parasitization showed inconsistent results i.e, prevalence of P. ophiocephali, E.heterostomum, S. visakhapatnamensis and L. bengalensis was more in females than the males whereas G. goppo and A. handiai showed more infection in males. Only males showed infection with L. chinensis (Table 8).
Co-existence of two species within a same host is referred it as interspecific association. Jaccard’s Index (JI) was employed to monitor interspecific association between each pair of parasite species. Existence of association between different species might be due to different habitat preference, sharing of same biotic and abiotic environments and mutual affinity for each other (Hubalek, 1982). From the above results it can be predicted that there is very less competition among species of ectoparasite and endoparasite in the host species as they share different niches within the hosts. Only, P. ophiocepali and G. goppo showed higher values suggesting that these parasites share a common niche i.e., intestine within the host (Table 9).
Hence, the present ecological study proposes that parasitic community of C. punctatus are less diverse, conventional, depauperate and non-interactive and holds good with the views of Homles (1990) who suggested that freshwater counterparts are less diverse than the marine ones.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest related to the work.
ACKNOWLEDGEMENTS
The First author, Anuprasanna Vankara is grateful to CSIR for providing the financial assistance as JRF and SRF.
AUTHOR’S CONTRIBUTION
The first author, Anuprasanna Vankara was involved in collecting the fish samples and parasites, literature collection, statistical analysis and written the manuscript while the second author, C. Vijayalakshmi was the research supervisor who guided in drafting the manuscript.
REFERENCES