The Journal of Advances in Parasitology
Research Article
Schistocephalus solidus (Cestoda: Ligulidae) Infection Characteristics and Pathology at the Highest Elevation Record Environment (Dalaman River Basin, Turkey)
SALİM Serkan GÜÇLÜ1, Ufuk Gürkan YILDIRIM1, DENİZ İNNAL2, Özlem ÖZMEN3
1Isparta University of Applied Sciences, Eğirdir Fisheries Faculty, Isparta, Turkey; 2Burdur Mehmet Akif Ersoy University, Department of Biology, Burdur, Turkey; 3Burdur Mehmet Akif Ersoy University, Faculty of Veterinary Medicine, Department of Pathology, Burdur, Turkey.
Abstract | This study was aimed to identify Schistocephalus solidus in three-spined stickleback, Gasterosteus aculeatus (Teleostei: Gasterosteidae) and to describe infection parameters and pathology. Three-spined stickleback specimens were collected between October 2019 to September 2020 in Gökpınar Spring (Dalaman River basin-Turkey). The highest elevation record of the common cestod parasite of three-spined stickleback has been found in this environment. Gross examination revealed that abdominal swelling was the most common finding. Cachexia and growth retartion were common in infected fishes. During the necropsy parasites were localized at the abdominal cavity. At the histopathological examination of the parasite, marked inflammatory reaction and fibrous tissue proliferation were observed. This study result showed that Schistocephalus solidus can cause marked pathological findings in infected three-spined stickleback.
Keywords | Anatolia, high altitude, pathology, three-spined stickleback, Cestoda
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | August 10, 2021; Accepted | September 14, 2021; Published | September 28, 2021
*Correspondence | Salim Serkan GÜÇLÜ. Isparta University of Applied Sciences, Eğirdir Fisheries Faculty, Isparta, Turkey; Email: salimguclu@isparta.edu.tr
Citation | GÜÇLÜ SS, YILDIRIM UG, İNNAL D, ÖZMEN O (2021). Schistocephalus solidus (cestoda: ligulidae) infection characteristics and pathology at the highest elevation record environment (dalaman river basin, turkey). J. Adv. Parasitol. 8(3): 32-36.
DOI | http://dx.doi.org/10.17582/journal.jap/2021/8.3.32.36
ISSN | 2311-4096
Copyright © 2021 GÜÇLÜ et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The Mediterranean region is a biodiversity hotspot for freshwater ecosystems, harboring many species (many of them endemic) and genetically distinct lineages that are of conservation concern (Araguas et al., 2012; Sharda et al., 2018). One of these species is the three–spined stickleback Gasterosteus aculeatus. It is a small teleost fish and is a model organism in evolutionary biology and ethology (Bell and Foster, 1994; Mäkinen et al., 2006; Cresko et al., 2007; Mäkinen and Merilä, 2008; Barber, 2013). It is distributed widely throughout the Holarctic region at latitudes ranging from 35° to 70° (Wootton, 1984).
During the past two decades, the three-spined stickleback (G. aculeatus), a small teleost easily reared in laboratory conditions, has become one of the most important model species in ecology and evolutionary biology (Fang et al., 2018). With a nearly circumpolar marine distribution in the Northern Hemisphere, this species has colonized freshwater habitats multiple times since the last glacial maximum, evolving dramatic morphological, physiological and behavioral adaptations in remarkably short periods of time (Barrett et al., 2011).
Sticklebacks are intermediate hosts to a number of parasites. Probably the most common, and certainly the most conspicuous of these is the large white, worm-like plerocercoid of the tapeworm Schistocephalus solidus (Maitland and Campbell, 1992). Schistocephalus solidus, a pseudophyllidean cestode, is threatening natural populations of G. aculeatus. This tapeworm belongs to the Cestoda family of obligate parasitic tapeworms. Copepods and three-spined sticklebacks (G. aculeatus) are the first and second intermediate hosts, respectively, and piscivorous birds are the definitive hosts (Clarke, 1954; Dubinina, 1980). Sticklebacks become infected by eating the copepod crustaceans that themselves heve been infected by ingesting the tapeworm eggs. These are released into the water from the faeces of a predatory bird, such as a gull, which is the final host-itself having been infected by swallowing an infected, probably disabled or dying, stickleback (Maitland and Campbell, 1992). The parasite has various effects on the physiology, phenotype, and behavior of its host (Hahn, 2020). The three-spined stickleback S. solidus host-parasite system has become an important model in experimental parasitology and is increasingly used to investigate a wide range of questions about host-parasite interactions and co-evolution (Barber and Scharsack, 2010).
Although plerocercoids of the S. solidus are common parasites of three-spined sticklebacks throughout the geographical range of the fish, there is no report from Turkey. There is little information regarding the parasitic infections of three-spined sticklebacks in different regions of Turkey (Özer, 2003; Öztürk and Özer, 2014; Öztürk and Özer, 2019). Schistocephalus solidus is a fairly common species; however, little is known about its altitudinal range. It lives in marine, brackish and inland water systems. The goal of this research is to add to our understanding of Schistocephalus solidus by reporting the species’ highest elevation record. This paper’s second goal is to give infection parameters. The third goal of this research is to assess the histological alterations that have occurred.
MATERIALS AND METHODS
The care of experimental animals was consistent with the Republic of Turkey animal welfare laws, guidelines and policies approved by Isparta University of Applied Sciences Local Ethics Committee for Animal Experiments (permit reference number 2020/001). The study was carried out in Gökpınar Spring (Dalaman River basin-Turkey) (37°20´37.3ʺN, 29°26´38.3ʺE - altitude: 850 m). Monthly samples were carried out in October 2019 and September 2020 with drift nets of tulle of 2 mm mesh size and 888 individuals were caught from Gökpınar Spring. Specimens of three-spined sticklebacks were placed in a well aerated 20 lt aquarium filled with stream water. The fish were maintained in the aquarium for 2-3 hours and subsequently anaesthetized by MS-222. The total length was measured and sex determined at necropsy by macroscopic investigation. During the dissection, abdominal cavity and visceral organs were examined separately under a dissecting microscope. Morphological identification of parasites was performed following by Chubb et al. (1987). The parasite has been identified as Schistocephalus solidus.
Prevalence and mean intensity were calculated by Bush et al. (1997). During the dissection fish samples totally fixed in 10% neutral buffered formalin for 2 days. After fixation fish samples were placed to the tissue processing cassettes. Then samples were routinely processed by an automatic tissue processor (Leica ASP300S, Wetzlar, Germany). For tissue processing, samples dehydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin wax. Afterward, serial sections of the paraffin blocks (5 μm thickness) were cut by using a rotary microtome (Leica RM 2155; Leica Microsystems, Wetzlar, Germany). Sections were stained with hematoxylin and eosin (HE) and examined using a light microscope. Microphotography was performed using the Database Manual Cell Sens Life Science Imaging Software System.
RESULTS
The total length of three-spined sticklebacks individuals varied between 1.38 and 7.08 cm, while the weight values ranged between 0.014 and 4.85 grams. Among the total number of 888 specimens of three-spined sticklebacks examined for parasite presence, 30 fish were infected with cestode Schistocephalus solidus (Figure 1, Table 1). The prevalence of infection was 3.38 % (sexes combined), while the mean intensity of infection during the study was 1.1 parasites per fish. The highest prevalence and mean intensity (5.83 % and 1.14 parasites/fish) was recorded in male host individuals (Table 1). Cestode infection was highest in summer (4.98%) and lowest in winter (1.41%) (Table 2).
Abdominal swelling was the most common finding during the gross examination. Plerocercoids were found in free form in the abdominal cavity. Generally, a single plerocercoid was encountered, while more than one parasite was found in some fish. The very prominent tegument of the plerocercoid was elongated like a sawtooth from front to back at longitudinal sections (Figure 2). At the transversal sections the outer layer of the parasite was smooth. Microscopic protrusions were observed on the tegument. At least 4 circularly and longitudinally muscle layers were observed from inner to outer side of the plerocercoid. On the serosal surfaces of the organs of the fish that touched the parasite, vascular hyperemia and inflammatory cell infiltrations, mostly of mononuclear cells, were observed (Figure 3).
DISCUSSION
Due to the importance of S. solidus for zoogeographic distribution of three-spined sticklebacks, this paper presents a new record of a specific cestode parasite of three-spined sticklebacks in Turkey. S. solidus has been found at various
Table 1: Prevalence and Intensity of infection in sex groups of G. aculeatus
| Sex |
TL-cm (min-max) |
W-g (min-max) |
N | N’ | P | Int. |
| Immature |
2.78 (1.38-3.96) |
0.26 (0.014-0.71) |
265 | 3 | 1.13 | 1 |
| Female |
5.05 (2.25-7.08) |
1.92 (0.14-4.85) |
233 | 6 | 2.58 | 1 |
| Male |
4.17 (2.44-6.38) |
0.96 (0.15-3.84) |
360 | 21 | 5.83 | 1.14 |
| Total |
3.99 (1.38-7.08) |
1.01 (0.014-4.85) |
888 | 30 | 3.38 | 1.1 |
N = total number of hosts examined; N’ = number of infected fishes; P = prevalence; Int = mean intensity of infection; TL= Total Length; W= Weight
Table 2: Seasonality of infection and mean infection intesity for S. solidus in G. aculeatus hosts
| Season | N | N’ | P | Total | Int. |
| Winter | 142 | 2 | 1.41 | 2 | 1 |
| Spring | 172 | 6 | 3.49 | 6 | 1 |
| Summer | 261 | 13 | 4.98 | 16 | 1.23 |
| Autumn | 313 | 9 | 2.88 | 9 | 1 |
| Total | 888 | 30 | 3.38 | 33 | 1.1 |
N = total number of hosts examined; N’ = number of infected fishes; P = prevalence; Int = mean intensity of infection

Figure 1: G. aculeatus individual infected with Schistocephalus solidus, 46.24 mm TL, May 2020 (S. solidus, circled in red.)
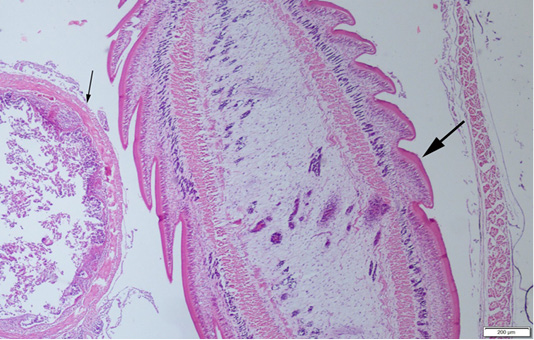
Figure 2: A plerocercoid (thick arrow) in abdominal cavity of the fish, near the intestine (thin arrow) of the host, HE, Bar=200µm.
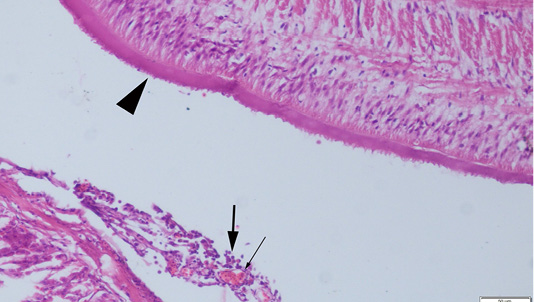
Figure 3: Serosal surface of the fish intestine, hyperemia of the vessels (thin arrow) and inflammatory cell infiltration (thick arrows) near the plerocercoid (arrow head), HE, Bar=50µm.
elevations. The parasite was found at the highest altitude in the globe in the research location.
The prevalence of S. solidus in three-spined sticklebacks in this study (3.38 %) is lower than from other studies performed in Palearctic region. Bergersen (1996) found from 18% to 92% infected sticklebacks in freshwater localities in Greenland. Zander (2007) found a maximum of 14% sticklebacks infected with S. solidus in the Baltic Sea. Prevalence of S. solidus in the three-spined sticklebacks collected from a Gdynia Marina (Poland) was 94.4% at 2008 (Morozińska-Gogol, 2011). The reported prevalence of S. solidus was 54.2% from Puck Bay (Mačát et al., 2015). Different prevalence values in S. solidus infections between the populations of three-spined sticklebacks may depend on the changing habitat conditions, physiology, immune parameters and health status of the fish. Different fish species infected with numerous parasites. Generally, hosts show no or little symptoms due to infection. Sometime clinical symptoms may occur at the tissue and organ level. Parasites can be localized all tissue or organ, but most common localization areas are abdominal cavity. Inflammatory reaction may occur if parasite remain in tissue for sufficient time. Common pathological findings were lymphocyte and macrophage infiltrations together with fibrous tissue proliferation around the parasite.
In this study, seasonal prevalence and histopathological findings in G. aculeatus caused by S. solidus was evaluated. The prevalence of the parasite was found higher in summer. At the histopathological examination all of the parasites were localized abdominal cavity. Parasites caused chronic inflammatory reaction characterized by mononuclear cell and macrophage infiltrations together with fibrous capsule around the parasites. This study revealed that S. solidus was the most common parasites of three-spined stickleback. This parasite caused clinical, gross and histopathological findings. The comparing the non-infected individuals infected ones were smaller and cachectic.
In fishes, parasitic infestations commonly occurs, prevalence of the infection may be higher in wild populations than cultured individuals (Roberts, 2012). Numerous parasites are reported in different fish species (Roberts, 2012). Pathology related the parasites, usually a combination of clinical signs, gross and histopathological findings of tissues and organs. Most of the parasites and reactions related the parasites can be identified during the hematoxylin and eosin stained sections (Bruno et al., 2006). Host responses against to parasites are important to evaluate parasite pathogenicity and it is generally assessed by histopathological examination (Yildiz et al., 2004). In addition, slight to marked abdominal swelling observed in infected fishes’ gross examination, this study finding indicated that S. solidus caused marked inflammatory reaction in three-spined stickleback. But the inflammatory reaction was commonly composed mononuclear cells indicating the chronical inflammation. These findings showed that this parasite can persist for a long time without causing fish death.
Parasitic infections are generally chronic and slightly effect the host (Feist and Longshaw, 2005; Bourque et al., 2006). Acute parasitic enfestations can cause high mortalities. Environmental factors such as temperature and dissolved oxygen concentration are the most important factors for parasitic infections (Feist and Longshaw, 2008). In this study, especially small number of parasites not caused the mortality according to gross and histopathological findings. Present study findings indicate that S. solidus may be cause reduced condition or slower growing rate but it were not highly fatal parasitic infection for Gasterosteus aculeatus.
The habitat for the study is spring water. For this reason, the water temperature is almost constant. The high rate of infection during the summer months depends on day length rather than water temperature. Besides, the presence of kingfisher (Alcedo atthis) in the habitat environment has been determined. Webster et al. (2011) stated that kingfishers are the predators of G.aculeatus, while Raven (1986) stated that G.aculeatus constitute the majority of the food of kingfishers. These data also pose the risk of migration of the parasite to different habitats through birds.
In conclusion, the first record of S.solidus being seen in three-spined sticklebacks in Turkey is given in this study. It was also identified as an infected at the highest altitude.
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
AUTHORS CONTRIBUTION
The authors contributed equally.
REFERENCES





