The Journal of Advances in Parasitology
Research Article
Morpho-metrical Observations on Trypanosoma evansi isolated from Slaughtered Camels in Assiut, Egypt and Study their Behavior Through Serial Mice Passages
Mohsen I. Arafa1, Gehan M. Sayed1, Wafaa G. Mahmoud2*
1Department of Parasitology, Animal Health Research Institute, Assiut Laboratory Egypt; 2Department of Parasitology, Faculty of Veterinary Medicine, New Valley University Egypt.
Abstract | A significant parasitic disease caused by Trypanosoma evansi (T. evansi) is camel trypanosomiasis. The present thesis attempts to explore the occurrence of T.evansi and its morphological forms in slaughtered camels in Assiut Governorate. As well as evaluating their behavior through serial passages of mice. Out of 64 slaughtered camels in Assiut Governorate during 2018- 2019, T. evansi was found in 10.93% (7/64) of examining camels by microscopical examination of blood smears. The infection was only detected in male samples as 12.82 % (7/57). Interestingly, several atypical forms and divisional features were observed in the blood smear of the highly parasitemic case. To describe the various forms of Trypanosoma in the current study, morphological and biometric measurements of T. evansi were taken. In vivo experiment through serial mice passages the infectivity and virulence of isolated T. evansi from infected camels were assessed .Pre-patent period, survival time and percentages of parasitemia were varied among different passages
Microscopic examination of blood smears of experimentally infected mice, revealed only trypanosome exclusively in the slender form with sporadic divisional features. Finally, described T. evansi in the present work was mostly exhibited as a slender form with very limited of polymorphism. Our experimental work provides information on the biological behavior of T. evansi in serial mice passages; which allows researchers to extend on experiments related to trypanosomes in the laboratory.
Keywords | Trypanosoma evansi, Camels, Polymorphism, Serial passages, Parasitemia
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | January 15, 2021; Accepted | February 26, 2021; Published | March 30, 2021
*Correspondence | Wafaa G Mahmoud, Department of Parasitology, Faculty of Veterinary Medicine, New Valley University Egypt; Email: wafaa3778@yahoo.com
Citation | Arafa MI, Sayed GM, Mahmoud WG (2021). Morpho-metrical observations on Trypanosoma evansiisolated from slaughtered camels in Assiut, Egypt and study their behavior through serial mice passages. J. Adv. Parasitol. 8(1): 1-8.
DOI | http://dx.doi.org/10.17582/journal.jap/2021/8.1.1.8
ISSN | 2311-4096
Copyright © 2021 Mahmoud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Trypanosoma evansi (T. evansi) is a haemoflagellate extracellular parasite causing a well-known disease termed surra, and its occurrence greatly influences livestock development (Luckins, 1988). T. evansi isn’t only spread by mechanical transmission by biting flies Tabanus spp. and Stomoxys spp., but also during nursing, copulation or ingestion of infected tissues by carnivores (Womak, 2001).
In countries with hot and mild temperatures and where camels are bred, surra is the major enzootic disease for camels with an incredibly large geographic range. (Eyob and Matios, 2013). It was reported in several Asian countries, African countries, South and Central America (Da Silva et al., 2010, Sow et al., 2014).T. evansi multiplies in a mammalian host by the process of longitudinal binary fission in the trypomastigote stage (Hoare, 1972).
In Egypt, T. evansi is a major livestock disease causing significant losses in camels (Abdel-Rady, 2008). There is also one rare case report of a human being infected with T. evansi in India (Joshi, et al., 2005).
Commonly, identification of T. evansi in stained blood smear based on the morphology and biometric details.It is a monomorphic trypanosome found exclusively on the slender intermediate types, although further studies have shown that on rare occasions, certain strains present stumpy forms (Hoare, 1972; Losos, 1980).
Intermittent parasitaemia is a widespread pattern of trypanosome infections,particularly T.evansi. Diagnosis of infected animals was traditionally based on parasite description in blood films (Nantulya, 1990).
Morales and Carreno (1976) was announced that T. evansi and T. brucei can produce related manifestations of the disease in laboratory animals.The abnormal hosts can often cause profound changes in the biology of trypanosomes in transmission experiments, including the production of dyskinetoplasty and subsequent growth patterns of normal and dyskinetoplastic types, contributing to the competitive exclusion of the later mutant morph (Misra, 1986).
Mice and rats have been mostly used for analyses pathogenic mechanism (Tarleton et al., 1992; Kierzeenbaum & Sztein, 1994) or to encourage the isolation of the parasite (Britto et al., 1996). Mice inoculation test considered more efficiency in the diagnosis of camel trypanosomosis especially in camels that show low number of parasites (El Sherif & Sayed, 2005). Conventional methods of keeping parasites require in vitro cultivation or frequent animal passages, which may cause alteration in virulence and/or genetic changes.
Mice are slightly less contagious with thoroughly frozen trypanosomes than non-treated organisms and their motility were strongly suppressed (Raether et al., 1988).
In the present work we describe the various morphological and biometric data corresponding to T. evansi isolated from slaughtered camels in Assiut. In addition to, study their behavior through serial mice passages.
Materials and methods
Samples collection
From different slaughter house inAssiut Governorate, 64 blood samples with EDTA were collected from slaughtered camels (57 males and 7 females) during 2018-201910 ml of jugular blood sample were collected from each animal under aseptic conditions. The samples were refrigerated and sent to the animal health laboratory Assiut, Egypt, for parasitological examination.
Examination of Blood for T. evansi
To detect motile trypanosomes, a microscopic inspection of the wet preparation of fresh blood was used. Also a drop of fresh blood was used for made thin blood smear and stained with Giemsa stain to identify parasites morphologically. Stained films were inspected carefully and measurements were taken using a light microscope (Soulsby, 1982). By oil immersion lens, digital light micrograph images were taken in the observed different stages of trypanosomes. Parasitaemia of positive blood of naturally infected camels was assessed according to Uzonna et al. (1998).
Morphometric Analyses
For biometric characterization, from each T evansi, three slides of stained thin films of blood were examined. Approximately 20 microscopic fields per slide were studied, and at least 10 trypanosomes were chosen for biometric study, measurements were occurred by eyepiece micrometer.
There were ten morphological parameters used: total length with flagellum (L), posterior end to kinetoplast (PK), posterior end to mid-nucleus(PN), kinetoplast to mid-nucleus (KN), mid-nucleus toanterior end (NA), free flagellum length (F), parasite maximum width (W), nucleus length (NL),in addition to the nuclear index (NI) and the kinetoplast index (KI)in compliance with the terms of morphometric values and generally agreed criteria (Hoare, 1972; John et al., 1992).
Experimental work
Infection of T. evansi in multiple mice passages: Twenty male albino mice were obtained from the Faculty of Veterinary Medicine at Assiut University, Egypt; with a mean weight of 30-35 g. Experimental housing and handling of the mice was conducted according to the guidelines of the Advisory Committee for Animal Experiments at animal health laboratory Assiut, Egypt and Faculty of Veterinary medicine at Assiut University, Egypt. Animals were tested for parasitic infection to prevent development of antibodies prior to experiments. They were kept in four separate clean cages and free access to drinking water and polluted food was permitted .After the proper dilution factor (1:10) of the infected blood sample, a standard inoculum dose as 0.2 ml containing 10³ of trypanosomes, was used for inoculation of laboratory mice.
Mice were divided into four groups A, B, C and D (five for each). On day 0, mice in group (A) were inoculated intraperitoneally with 0.2 ml- infected blood (10³ trypomastigotes). At peak parasitaemia of first passage (A), one animal was anaesthetized (the others were kept as backup) its blood was collected in heparin, diluted and inoculated into the next sets of animals (B) for the second passage.
Table 1: Measurements of Trypanosoma evansi of examined camels.
| Parameter (µm) |
Mean (μm)
± SD |
Range | |
| Maximum (μm) | Minimum (μm) | ||
| TL (µm) | 29.4 ± 3.9 | 33.7 | 23.8 |
|
BW (µm) |
2.8 ± 0.7 | 3.6 | 1.5 |
|
PK (µm) |
2.2 ± 0.7 | 2.5 | 0.0 |
|
KN (µm) |
8.3 ± 1.6 | 11.5 | 5.0 |
|
PN (µm) |
10.4 ± 1.9 | 13.0 | 7.2 |
|
NA (µm) |
8.1 ± 1.98 | 12.0 | 5.1 |
|
FF (µm) |
10.49 ± 2.7 | 15.5 | 4.8 |
|
NL (µm) |
2.6 ± .6 | 3.5 | 1.8 |
|
KI (PN/KN) |
1.27 ± 0.7 | 1.6 | 1.0 |
|
NI (PN/NA) |
1.3 ± 0.2 | 1.7 |
0.9 |
(TL) total T. evansi length, (BW) body width of T. evansi, (PK) distance between kinetoplast and posterior end, (KN) distance between kinetoplast and middle of the nucleus, (PN) distance between posterior end and middle of the nucleus, (NA) distance between middle of the nucleus and anterior end, (FF) length of the free flagella (NL) length of nucleus, (NI) nuclear index, (KI) kinetoplastic index.
Table 2: The pre-patent period, survival time, peak and mean of parasitaemia of infected laboratory animals.
| Items | Passage Groups | P-Value | ||
| First | Second | Third | ||
| Pre-patent period | 18 h | 24 h | 3 days | - |
|
Survival time |
13 days | 19 days | 25 days | - |
|
Parasitaemia (peak) / 100 RBC |
34 | 2.3 | 4.2 | - |
|
Parasitaemia (Mean) |
16.68a ± 3.64 |
1.03b ± 0.19 |
1.42b ± 0.26 |
0.0001 |
Means within the same row bearing different superscripts differ significantly (p<0.05).
The same steps were repeated to induce the infection from (B) into animals of the third passage (C) while; group (D) was kept uninfected as a control group.
Daily follow-up to establish the pre-patent duration, patent period and peak of parasitemia, a regular analysis of the blood of laboratory infected animals was performed. Wet smears as well as thin blood films were checked daily for trypanosomes according to Brener (1962). T. evansi trypomastigotes were counted in 10 fields and the mean of these counts was estimated to express the level of parasitemia by the number of trypomastigotes in a high-power field (Uzonnaet al.,1998).
Statistical analysis
The results of parasitemia in all passages were analyzed statistically using SAS statistical program (SAS, 2001). The data were evaluated by using the General Linear Models (GLM) procedure for analysis of variance. The results were subjected to one-way ANOVA accompanied by Duncan’s multiple range tests to detect the differences among the treatments. The data are presented as means ± SE. Probability values of less than 0.05 (p < 0.05) were considered to be significant.
Results
Prevalence of trypanosomiasis
Out of 64 camel blood samples were microscopically examined, T. evansi was detected in 10.93% (7/64) of examining camels. The infection rate in males was 12.28% (7/57) while it was not detected in females. Mean of parasitemia of T. evansi in blood smear of infected camels was 30.8 (3-108/1000 RBC).
Morphological characters of T. evansi
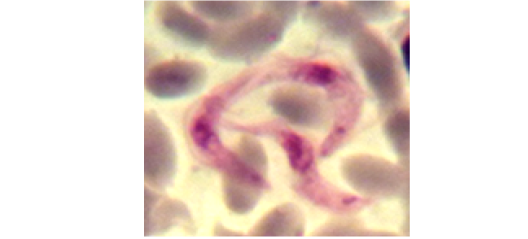
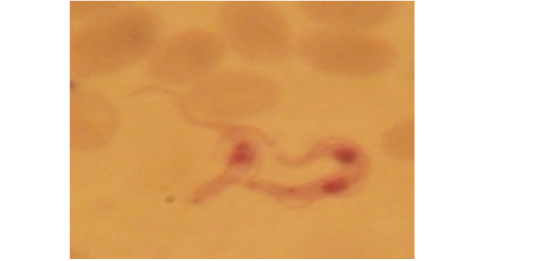
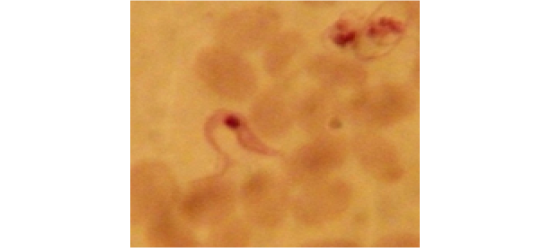
Examination of the blood films of infected camels had low parasitemia revealed only monomorphic trypanosome exclusively in the slender forms (Fig. 1 & 4). In highly parasitemic case T. evansi were polymorphic trypanosome showing three distinct forms slender, shorter intermediate and stumpy forms (Fig 2 & 3) also blood films were abundant with divisional stages included presence of two kinetoplast, two flagella or two nuclei (Fig 4- 6). Uncommon shape alterations by T. evansi were also observed included amastigote(a divisional stage appeared as round forms without a free flagellum), sphaeromastigote (a divisional stage appeared as flagellate round forms with a flagellum running around the organism) and dyskinetoplastic forms (Fig. 7- 9).

Figure 4: Showing divisional features of T. evansi i.e.; presence of two kinetoplast in examined camel (x 1000).
Biometrical analysis of T. evansi
Morphometric; measurements of different forms of T. evansi detected in the present work ; slender trypanosomes including the free flagellum was 23.8 – 33.7 x 1.5 – 3.6 μm, intermediate form12- 21 x 2 - 3.5 μm., stumpy form12.5 – 17 x 2.8- 2.5μm., sphaeromastigote 8.5 - 12 x 2 - 3 μm. and amastigote 2 - 4 x2 μm (Table 1).
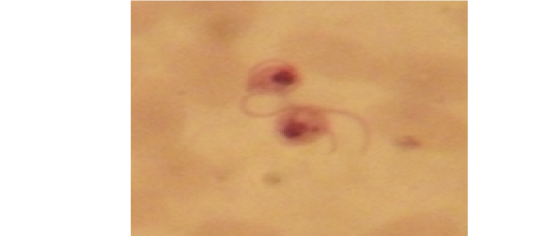
Figure 7: Showing uncommon shape alterations were documented; sphaeromastigote of T. evansi in examined camel (x 1000).
The biometrical indices [nuclear index (NI) and kinetoplastic index (KI )] of investigated T. evansi slender trypomastigotes were characterized by the following; their nucleus were mostly located in the anterior half of the trypomastigote body where the NI was 1.3 µm (ranged 1.7–0.9µm) and their kinetoplast were frequently sub terminal where the KI was 1.27 µm (ranged 1.0–1.6 µm).
Pattern of Infection in Different Mice Passages
Isolate of T. evansi in the present work was extremely virulent for this breed of mice; since all inoculated mice contracted infection. Observations of inoculated mice showed variation in the prepatent period, the level of parasitemia and survival time as shown in Table (2). Group D (non-infected group) showed no disease problems until the end of the experiment.
The results in Table (2) shown that the parasitaemia was significantly higher (P˂0.05) in the first passage than second and third passages (16.68 vs. 2.3 and 4.2), respectively. However, no significant differences were detected between second and third passages.
First passage: The average of firstly observation of trypanosomes in the peripheral blood (prepatent period) was 18h post inoculation. Initially, there were only a few trypanosomes, and from the 4thday onwards an increase of the parasitemia was detected, which reached the highest level during the period between 5-7 days post inoculations with a maximum average 24.8/ 100 RBC. Lowering of parasitemia was seen from 8-10 days post inoculation. So, their parasitemia soon escalateduntil the animals died on the 12thto13thdays post inoculation where the mean of parasitaemia was 34/ 100 RBC. Population density of trypanosomes in the blood showing two peaks the first at sixth day and the second peak just before death at thirteen day. None survived beyond the fourteenth day where mortality rate was 100% (Figure 10).
Second passage: The trypanosomes were first observed in the peripheral blood after one days post inoculation Curve of parasitemia in infected mice was intermittent; there was a sudden drop in the mean count of trypanosomes of 8-9thdays post inoculation and at 12-14thdays post inoculation during these periods no circulating parasites were microscopically seen. Estimation of the parasitemia level in second passage was much lower than that observed in mice during first one which were not more than 2.3/ 100 RBC. There are three peaks of parasitemia at 5th, 10th and 18thdays post inoculation Infected mice died within 17- 20 days post inoculation (Figure 10).
Third passage: Similar to the second passage infected mice in the third passage showed lower and fluctuating parasitemia curve that resembles waves for 24 days. The average of pre-patent period was 3–4 days thereafter, blood remained negative for 2-3 days. There are three peaks of parasitemia which were not more than 4.2/ 100 RBC. Trypanosoma disappears from peripheral blood four times at 6, 16th18thand 20 days post inoculation one survived beyond the twenty four days after the fourth peak of parasitemia (Figure 10).

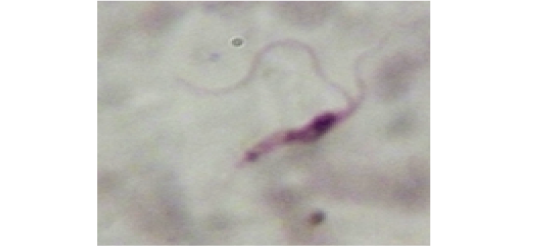
Figure 11: Showing slender form of T. evansi in blood of infected mouse and divisional stage characterized by presence of two kinetoplast and two nuclei. x1000.
Stained blood smear preparations in different passages of infected mice showed that all detected trypanosomes in the blood were monomorphic where the only slender shape was found. The mean length of the parasite is 27±2µm (min 24µm, max 30µm). Few trypanosomes were found to multiply in the peripheral blood, especially in case of high parasitaemia (as shown in Figure 11).
Discussion
Protozoan parasite T. evansi is the causative agent of trypanosomiasis in camels which called (Surra) which considered the most common endemic protozoan disease of camels and various domestic animals in the world (Shahid et al., 2013).
Based on the taxonomic data of previous research work (Hoare, 1972; Soulsby, 1982; Desquesnes, 2004) trypanosomes parasite in the present investigation was established as T. evansi based on morphological and morphometrical data.
The prevalence of trypanosomiasis in the present study among slaughtered camels in Assiut was 10.93% (7/64).The infection rate in males was 12.28% (7/57) while T. evansi was not detected in females.
This infection rate appears to be low which may be as a result of periodical injected camels with anti-trypanosomiasis as a routine protocol precaution method of camel rearing. Also, the rate of infection is affected by the rainy season, which influences on the presence of their vectors and thus affects the infection rates of T. evansi.
The lower infection rate in the present work (10.93%) was more or less coincided with the previous results in Egypt; Mehran (2004) 11.5% in Shalatin city –Red sea Governorate, and Elhaig et al.(2013)12% in Ismailia, El-Hewairy et al. (2014) 16.9% in Abu-simbuel and Darwa city –Aswan Governorate. Much lower infection rate of T.evansi in camels was recorded by Abdel-Rady (2008) 4.1% in Aswan and Sohag Governorates, Zayed et al. (2010) 5.67% infarm animals in Egypt.
On the other hand, high prevalence of T.evansi in camels in Egypt was detected previously by El Sherif & Sayed (2005) 36.31% also Mahmoud et al. (2008) 27.6% in southern area of Egypt.
The variation in the rate of infection may be due to the variation in environmental and climate dynamics that support fly vectors indifferent regions, the existence of reservoirs and susceptible hosts in one pasture, the breeding system, the quarantine measures and the diagnostic procedures used. (Alarabi et al., 2019).
Regarding sex, we could not assess the effect of sex on the prevalence of T.evansi as a result of the low number of slaughtered females during the period of samples collection, where most of examined camels were males.
Different biometrical indices in the present work showed that T. evansi were characterized mostly by a slender body, nucleus located in the anterior half, and sub-terminal kinetoplast. All our isolates ranged 23.8–33.7µm length, as described by Hoare (1972) who reported the morphometric total length of T. evansi ranged between 15 and 34 μm, and the nucleus positioned in the anterior part of the trypanosome. Our mean morphological parameters (L, PN, NA, F, W and NI) were compatible with the data obtained in T. evansi by other authors (Silva et al., 2002; Sarataphan et al., 2007).
T.evansi is morphologically monomorphic, represented by the predominant long, slender forms of its trypomastigote phase. Some stumpy forms, intermediate and short, correspond to those of T.brucei has been recorded (Hoare, 1972). In the present investigation some degree of polymorphism was observed in highly parasitimic case. There have been records of a small range of various forms, such as short stumpy, intermediate, sphaeromastigote, mastigote and dyskinetoplaste forms. These results agree with several studies observed significant morphological differences in some T. evansi isolates (Dávila et al., 1998; González et al., 2003; Khalafalla and Mawly, 2020).While, Lai et al. (2008) mentioned that originally the term dyskinetoplastic described trypanosomes completely lacking a kDNA structure. Schnaufer et al. (2002) added that k DNA is fragile, highly sensitive to drugs and is sometimes altered or even lost in nature, giving rise to induced and natural dyskinetoplastic (Dk) strains of trypanosomatids, respectively.
Dávila et al. (1998) claimed that the shorter forms of T.vivax may be linked with the acute diseasefound in blood films from spontaneously infected bovine animals. Seed & Black (1997) reported that a population density the reason for the transition from long slender form to short stumpy form. Tejero et al. (2008) on the other hand, documented a relationship between the detectable morphology of the parasite and the physical state of the host.
In the present experimental work, changes in the biological behavior of T. evansi were noted after been retained in numerous mice passages for some time. Results in the present work showed differences in pre-patent period, survival time and mean parasitaemia of three mice passages. Parasitaemia fluctuations have arisen in all affected populations. The higher parasitaemia in the first mice passage may relate to virulence of isolate strain of T. evansi and their multiplication by binary fission, so the concentration rises exponentially. This result was confirmed by Ukoli (1984), who noted that the ability of the parasite to alter its surface coats contributing to the production of new forms of antigenic that may not be influenced by the prevalent host antibodies which lead to the fluctuating parasitaemia.
Short survival time and the rapid death of the first passage can be due to rapid dissemination of virulent isolate which translate into energy requirements that surpass the host’s capacity. Bal et al. (2012) mentioned that trypanosomes use the host’s glucose and oxygen for their growth and multiplication, resulting in the degenerative changes in the host. The high mortality rate ofinfected mice in different passages (up to 100%), indicating that mice appeared to be highly susceptible to infections. The sensitivity of tiny rodents such as mice to degenerative changes during trypanosome infectionmay be due to parasite-released toxins or the host’s immunological reactions to the infection. (Bal et al., 2012). On the other hand, host immune responses during infection do not contribute to defense, but are implicated in immunopathological disturbances that can lead to tissue changes (Vincendeau and Bouteille, 2006).
There was a considerable extension of life span of infected mice and appreciable decline in parasitaemia in second and third passage compared with the first one. This may refer to a certain degree of attenuation in inoculated trepanosomes. Raether et al. (1988) mentioned that frequent animal passages which used as a conventional method for keeping of trypanosomes may cause alteration in their virulence and/or genetic changes.
While, Pérez, et al. (2011) mentioned that survival time of infected animals may possibly be attributed to the host’s improved IgG antibody production and estradiol levels.
In the present work even though inculcated specimen was pleomorphic strain, microscopic examination of blood smears of all experimentally infected animals revealed only monomorphic forms of T. evansi. Misra et al. (2016) mentioned that polymorphism in T. evansi is not persistent. They added that if syringe passage to rodent hosts is a polymorphic strain, T.evansi flipped into a monomorphic form.
Conclusion
Morphometrically described T. evansi in the present work was mostly exhibited as a slender form with very limited of polymorphism. Experimentally, it is believed that multiple passages of trypanosomes in micelead to alterations on their virulence. In order to understand the mechanism of potential impact on trypanosome virulence across numerous passages in laboratory animals, further studies would be required.
Conflict of interest
The authors declare that there is no conflict of interest.
authors contribution
All authors contributed equally.
References