The Journal of Advances in Parasitology
Research Article
The Journal of Advances in Parasitology. 1 (1): 9 – 11Prevalence of Ecto and Gastrointestinal Parasitic Infections of Pigeon at Chittagong Metropolitan Area, Bangladesh
Kazal Krishna Ghosh1*, Muhammad Shafiqul Islam2, Suchandan Sikder3, Shubhagata Das2, Sharmin Chowdhury2 Muhammad Abdul Alim2
- Department of Microbiology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong–4225
- Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong–4225
- Department of Medicine and Surgery, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong–4225
*Corresponding author: kazal_krishna@yahoo.com
ARTICLE CITATION:
Ghosh KK, Islam MS, Sikder S, Das S, Chowdhury S and Alim MA (2014). Prevalence of ecto and gastrointestinal parasitic infections of pigeon at Chittagong metropolitan area, Bangladesh. J. Adv. Parasitol. 1 (1): 9 – 11.
Received: 2013–12–24, Revised: 2014–01–03, Accepted: 2014–01–06
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.jap/2014/1.1.9.11)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
A study was conducted to assess the prevalence of ecto and gastrointestinal parasitic infections in domestic pigeons (Columba livia) in Chittagong Metropolitan area, Bangladesh. A total of 100 pigeons were examined for the presence of ecto and gastrointestinal (GI) parasitic infections. The investigation revealed that the overall prevalence of ecto–parasitic infestation was 67%. Among 6 different ecto–parasites, the highest infestation was caused by Pseudolynchia canariensis (43%). It was also explored that pediculosis was a common problem in the study population where Lipeurus caponis (28%) and Goniodes gallinae (28%) occurred more frequently as compare to Menopon gallinae which constituted the lowest 13%. Further, adult pigeons (86%) were more prone to ecto–parasitic infections in compare to squabs (48%), which was statistically significant (P<0.05). Conversely, the overall prevalence of gastrointestinal parasitic infections was 72% (single or mixed). Among six different species, the highest prevalence was recorded for Ascaridia galli (35%) followed by Capillaria sp infection (22%). Moreover, association between occurrance of gastrointestinal parasitic infections with the stages of the pigeon showed that adults (74%) were more susceptible to GI parasitic infections in compare to squabs (70%) but it was not statistically significant (P>0.05). Among GI parasites, occurrence of Ascaridia galli (32%) and Capillaria sp infections (26%) were more common in adult pigeon where as Raillietina sp (0%) and Syngamus trachea infections (2%) were less frequent in squabs. Finally, it gave an overall idea about the distribution of ecto and GI parasites in association with stage or age of pigeon. We recommended further extensive study which will ultimately assist to take necessary preventive measures against such diseases.
INTRODUCTION
Pigeon of the order Columbiformes are ubiquitous bird and can be found in virtually every town and city around the world (Marques et al., 2007). Pigeons have been accompanied by human since ancient time. They live side by side with human as a source of food, hobby and experimental purposes (Sari et al., 2008).
Several health problems can affect pigeon where ecto–and endo–parasitic infections play a major role (Marques et al., 2007). Among different gastrointestinal (GI) helminthes, nematodes are the most deleterious parasites responsible for occurrence of clinical and sub–clinical parasitic diseases. Moreover, they are also severely affected by ecto–parasites which are responsible for nuisance, anemia, general debility and some of them have a significant role in the transmission of various infectious agents. In general, the losses caused by both ecto and endo parasitic infections are in the form of lowered general health condition, retarded growth rate, unthriftiness, production loss, cost associated with therapeutic and preventive measures and also increase susceptibility to other infectious diseases which may ultimately lead to higher mortality of the pigeons (Urquhart, 1996).
Several research works have been conducted on parasitic diseases of pigeon at different areas of Bangladesh but limited works conducted at Chittagong region. Therefore, the present study was designed to determine the ecto and gastrointestinal parasitic infections of domestic pigeon at Chittagong Metropolitan Area (CMA). The investigation will give an overall idea about the distribution of ecto and gastrointestinal parasitic infections of pigeon of this region which will ultimately assist the clinicians regarding epidemiological forecasting and aware the farmers to take appropriate measures against them.
MATERIALS AND METHODS
Study Area
The study was conducted for a period of five months starting from August to December’2012. During this investigation, a total of 100 birds (50 adults and 50 squabs) were considered from 4 different farms located at four different areas (Khulshi, Kornelhat, Agrabad and Oxygen) of CMA, Bangladesh.
Sample Collection and Examination
A prototype questionnaire was designed to collect the objectives oriented data from each bird. Two types of biological samples; ecto–parasites and faeces were collected from the sampled pigeon. For the collection of ecto–parasites, aerosol was sprayed over the feathers of the body and left for five minutes. After shaking the pigeon, parasites were collected and preserved in 70% alcohol. However, fresh fecal sample were collected and preserved in 10% formalin. Later, all the samples were brought to the Parasitology laboratory of Chittagong Veterinary and Animal Sciences University (CVASU) for further examinations. Finally, the ecto–parasites were identified according to Wall and Shearer (1997); Soulsby (1982); Kettle (1995). On the other hand, three different types of qualitative tests; namely direct smear, flotation and sedimentation techniques were used to examine the fecal samples to identify the morphological features of eggs, cyst, Oocysts (Hendrin and Robinson, 2006; Soulsby, 1982).
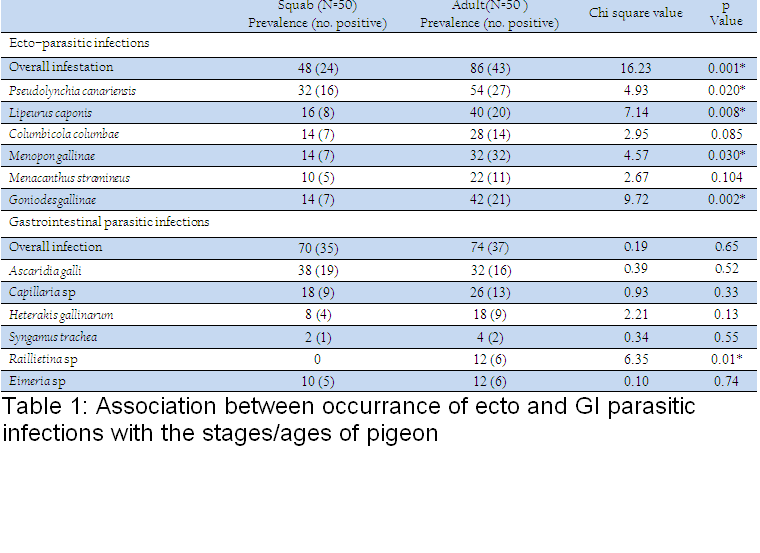
Table 1: Association between occurrance of ecto and GI parasitic infections with the stages/ages of pigeon
Sample Collection and Examination
A prototype questionnaire was designed to collect the objectives oriented data from each bird. Two types of biological samples; ecto–parasites and faeces were collected from the sampled pigeon. For the collection of ecto–parasites, aerosol was sprayed over the feathers of the body and left for five minutes. After shaking the pigeon, parasites were collected and preserved in 70% alcohol. However, fresh fecal sample were collected and preserved in 10% formalin. Later, all the samples were brought to the Parasitology laboratory of Chittagong Veterinary and Animal Sciences University (CVASU) for further examinations. Finally, the ecto–parasites were identified according to Wall and Shearer (1997); Soulsby (1982); Kettle (1995). On the other hand, three different types of qualitative tests; namely direct smear, flotation and sedimentation techniques were used to examine the fecal samples to identify the morphological features of eggs, cyst, Oocysts (Hendrin and Robinson, 2006; Soulsby, 1982).
Statistical Analysis
Obtained data were analyzed by using statistical software ‘STATA®’ (version 9.2). Chi–Square Test were performed and the results were expressed in percentage with p–value and significance was determined when P<0.05.
RRESULTS
Prevalence of Ecto and Gastrointestinal Parasites
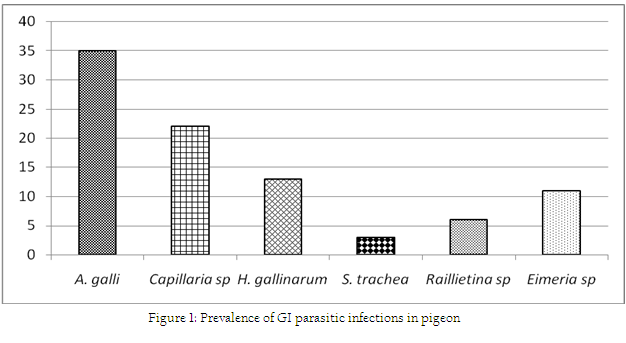
Association between occurrance of ecto and GI parasitic infections with the stages/ages of pigeon has been shown in Table 1. During this investigation, 6 species of ecto–parasites were identified and the overall prevalence of ecto–parasites was 67%. The highest infestation was caused by Pseudolynchia canariensis (43%). Among different lice, Lipeurus caponis (28%) and Goniodes gallinae (28%) were the most common causes of pediculosis in the study population. Further, Columbicola columbae (21%) and Menacanthus stramineus (16%) were moderately prevalent species. The least common causes of pediculosis were caused by Menopon gallinae which constituted only 13%. On the other hand, the overall prevalence of GI parasitic infections was 72% (single or mixed infection) (Fig. 1). The current investigation identified six different types of gastro intestinal parasitic infections in the study population where four species belongs to nematode. Cestode and enteric protozoan infection were less frequent in the study population.
DISCUSSION
Prevalence of Ecto–Parasitic Infestations
The documented overall prevalence of ecto–parasitic infestations of the current study showed similarity with the report of Msoffe et al. (2010). The observations of this study somewhat lower the report of Musa et al. (2011). The higher prevalence of ecto–parasitic infections might be due to due to favorable climatic conditions as well as diversified topography of the sampled areas (combination of plane, hilly and coastal areas) which favors the growth and development of such ecto–parasites. Moreover, the occurrence of Pseudolynchia canariensis showed similarities with the reports of Msoffe et al. (2010) who recorded 61.5% in Tanzania. The earlier observation of this study showed discrepancy with findings of Dranzoa et al. (1999) who recorded higher prevalence (100%) in Uganda.
Furthermore, pediculosis was another common problem in adult pigeon as compare to squabs which was in accordance with the findings of Msoffe et al. (2010). Adult pigeon had the easy access to free range area and got more contact with other birds which might be the cause of higher prevalence of such infestation in the study population. The higher prevalence of such ecto–parasites also indicated a continuous trend of such infections which contributed in transmission of different infections among pigeons as they act as significant mechanical or biological vectors.
Prevalence of Gastrointestinal Parasitic Infections
The overall prevalence of GI parasitic infection of the current study was in line with the findings of Marques et al. (2007). The earlier observation was lower than the report of Parsani and Momin (2010) and Begum and Shaikh (1987) who found 88.88% and 86% nematode infection, respectively. Moreover, higher prevalence of Ascaridia galli infection showed the similarity with the observation of Rabbi et al. (2006). Similarly, occurrence of Capillaria sp and Heterakis gallinarum were much lower than the observation made by the earlier author. Moreover, the lowest prevalence of Syngamus trachea infection of the present found similar with the findings of Sari et al. (2008). Frequent occurrence of such nematode infection of this study might be due to higher resistant of those eggs to common disinfectants and other environmental extremes (Soulsby, 1982). On the other hand, the overall infection rate of gastrointestinal parasitic infections was higher in adults in compare to squabs. Similarly, occurrences of GI parasitic infections were more frequent in adults than squabs. But, Ascaridia galli infection was slightly higher in squabs which showed similarities with the report of Msoffe et al. (2010). Higher prevalence of such parasitic infections in adult pigeon might be due to sharing of common premises with other poultry species. These contaminated premises or soil acts as an important reservoir and transmission media for soil transmitted helminthes (Islam et al., 2009).
CONCLUSION
The study was performed to determine the prevalence of ecto and gastrointestinal parasitic infections in pigeon of CMA. The explored information of this study will give an overall idea about the distribution of ecto and gastrointestinal parasitic infections among the study areas. It will also provide some epidemiological ideas in the occurrence of such diseases. However, this study will make the way to take further extensive study related to these infections which will help to take necessary preventive and control measures against them.
ACKNOWLEDGEMENT
We greatly acknowledged to Professor Dr. Md. Masuduzzaman, Head, Department of Pathology and Parasitology, CVASU, Bangladesh for his kind support to conduct this research.
REFERENCES
Begum NJ and Shaikh H (1987). Prevalence of helminth parasites of pigeons (Columba livia). Bang. Vet. J. 21: 89–93.
Dranzoa C, Ocaido M and Katete P (1999). The ecto, gastro–intestinal and haemo parasites of live pigeons (Columba livia) in Kampala, Uganda. A. Path. 28: 119–124.
Hendrin CM and Robinson N (2006). Diagnostic Parasitology for Veterinary Technicians (3rd Ed.), Mosby Inc. and affiliated of Elesevier Inc., 255–260.
Islam A, Trisha AA, Das M and Amin MR (2009). Retrospective study of some poultry diseases at Gaibandha district in Bangladesh. Bang. J. Vet. Med. 7(1): 239–247.
Kettle DS (1995). Medical and Veterinary Entomology (2nd Ed), CAB international, 371–376.
Marques SM, Quadros RM, Da–Silva CJ and Baldo M (2007). Parasites of pigeon (Columba livia) in urban areas of lages, Southern Brazil. Para. Lati. 62: 183–187.
Msoffe PLM, Muhairwa AP, Chiwanga GH and Kassuku AA (2010). A study of ecto– and endo–parasites of domestic pigeons in Morogoro Municipality, Tanzania. Afri. J. Agri. Re. 5(3): 264–267.
Musa S, Afroz SD and Khanum H (2011). Occurrence of Ecto and Endo Parasites of pigeon (Columba livia linn.). Univ. J. Zool. Rajshahi Univ. 30: 73–75.
Parsani HR and Momin RR (2010). Prevalence of Nematode infection of pigeons of Gujarat state, India. Zoo,s Print(web version). 25(10): 32–34.
Rabbi AKMA, Islam A, Majumder S, Anisuzzaman and Rahman MH (2006). Gastro intestinal helmiths infection of poultry. Bang. J. Vet. Med. 4(1): 13–18.
Sari B, Karatepe B, Karatepe M and Kara M (2008). Parasites of domestic pigeon (Columba livia domestica) and wild (Columba livia livia) pigeons in Nigde, Turkey. Bull. Vet. Inst. Pulawy. 52: 551–554.
Soulsby EJL (1982). Helminths, Arthopods and Protozoa of domesticated animals (7th Ed), ELBS and Bailliere Tindall, London.
PMCid:PMC370254
Urquhart GM (1996). Veterinary Parasitology (1st Ed), ELBS, Longman House, Burnt Mill, Harlow, England, 256–257.
Wall R and Shearer D (1997). Veterinary entomology (1st Ed), Chapman and Hall, London, UK, 284–311.
http://dx.doi.org/10.1007/978-94-011-5852-7_7
http://dx.doi.org/10.1007/978-94-011-5852-7