The Journal of Advances in Parasitology
Research Article
Coproscopic and Slaughter House Study of Paramphistomiasis in Cattle at Sylhet Division of Bangladesh
Tamanna Jahan Chowdhury1, Md. Taslim Hossain2, Selina Akhter1, Md Bashir Uddin1, Md. Shahidur Rahman Chowdhury1, Md. Mahfujur Rahman1, Md. Mukter Hossain1*
1Department of Medicine, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet-3100, Bangladesh; 2Youth Training Center, Department of Youth Development, Ministry of Youth and Sports, Bangladesh.
Abstract | To investigate the present status of paramphistomum infection in cattle by coproscopy and slaughter house study, fecal samples were collected from different areas and slaughter house survey was conducted during the period of September 2016 to August 2017. A total 613 of feces and 215 of slaughtered animals were examined of which 226 (36.87%) and 196 (91.16%) were found to contain paramphistomum spp. In fecal examinations, age and sex of animals significantly (P<0.05) influenced the prevalence of paramphistomiasis. Older animals suffered (54.55%) more than growing (36%) and young (30.82%) ones. Furthermore, females were more (39.46%) susceptible to paramphistamum spp. than males (27.13%). The prevalence of paramphistomiasis in cattle varied depending on the season of the year; being highest in the rainy season (40.71%) followed by the summer (37.30%) and winter (34.68%). Prevalence of paramphistomiasis was found highest in Sunamganj district (52.31%) and lowest in Sylhet district (32.41%). In slaughter house study, similar findings were observed as coprology. Females (93.33%) were more infected than males (90.81%). Older animals were (92.72%) more susceptible than young animals (90.63%). Prevalence was highest in rainy (95.40%) followed winter and summer of 93.33% and 87.61% respectively. This study revealed the infection of paramphistomum is prevalent in cattle of different districts at Sylhet division. Therefore, further studies with continuous monitoring of the infection and appropriate control strategy need to be established.
Keywords | Paramphistomiasis, Prevalence, Coproscopy, Slaughter house, Cattle
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | June 26, 2019; Accepted | July 05, 2019; Published | September 20, 2019
*Correspondence | Md. Mukter Hossain, Department of Medicine, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet-3100, Bangladesh; Email: mukter.vetmed@sau.ac.bd
Citation | Chowdhury TJ, Hossain MT, Akhter S, Uddin MB, Chowdhury MSR, Rahman MM, Hossain MM (2019). Coproscopic and slaughter house study of paramphistomiasis in cattle at sylhet division of bangladesh. J. Adv. Parasitol. 6(3): 35-40.
DOI | http://dx.doi.org/10.17582/journal.jap/2019/6.3.35.40
Copyright © 2019 Hossain et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Paramphistomiasis is a parasitic disease of livestock animals, more commonly of cattle, sheep and humans caused by immature helminthes belonging to the family Paramphistomatidae. Most paramphistomes are responsible for the disease in livestock animals (Phiri et al., 2006; Hugh-Jones et al., 2008). Paramphistomum cervi is considered to be one of the most important species since they are cattle parasites with cosmopolitan distribution (Ilha et al., 2005). There are 83.9% of total households in Bangladesh own livestock about 45.9% households possess bovine stack and on average each households own 1.52 bovine animals.
In Bangladesh, there are many constrains in cattle production, among them malnutrition and parasitism are the major limiting factors (Jabber and Green, 1983). Adult paramphistomes are one of the parasites in the rumen and reticulum of sheep, goats, cattle and water buffaloes (Ozdal et al., 2010). It has been estimated that more than 500 million cattle worldwide are at risk due to parasitic infection (Juyal et al., 2003; Ilha et al., 2005). The geo-climatic conditions together with the water-logged and low-lying areas in Bangladesh are conducive to parasitic diseases in domestic ruminants. In fact, cattle of Bangladesh are affected by various types of helminthic parasites (Rahman and Mondal, 1983). However, fecal examinations of cattle and buffaloes from some limited areas of Bangladesh have also suggested that the prevalence of Paramphistomiasis is very much common in Bangladesh (Rahman and Mondal, 1983; Afazuddin, 1985; Saifuzzaman, 1996). In ruminants, paramphistomiasis is often associated with diarrhea, loss of body condition, rough hair coat, dullness, weakness, loss of appetite, intestinal hemorrhages, anemia, reduced milk production and inter-mandibular swelling (Chandrasekharan et al., 1982). Light infection does not cause serious damage to the animals, but massive number of immature paramphistomes can migrate through the intestinal tract causing acute parasitic gastroenteritis with high morbidity and mortality rates, particularly in young animals (Hanna et al., 1988). Death due to immature paramphistomes is very high and may be as high as 80-90% in domesticated ruminants causing heavy economic losses amounting several thousand crores rupees annually (Juyal et al., 2003; Ilha et al., 2005). However, the epidemiological study of Paramphistomiasis has not yet been investigated properly in cattle at the different areas of Sylhet division in Bangladesh. Therefore, this study was undertaken to estimate the prevalence of paramphistomiasis by using coproscopy and slaughter house as a survey tool. Egg identification from fecal sample is a usual tool to investigate prevalence of paramphistomiasis. In this study gross examination of visceral organ (rumen and reticulum) of the slaughtered animals has been done in different slaughter house by close inspection. Domestic ruminants chronically infected are responsible for the spread of the disease by contaminating the pastures with fluke eggs; this is especially seen in areas that have favorable climatic conditions and suitable intermediate hosts. For prevention or decrease in number of this infection, identification of present status of the parasite is necessary. Therefore, in this study, an attempt was made to the present status of infection in cattle associated with the influence of age and to correlate among other factors related with the parasitism.
MATERIALS AND METHODS
Study Area and Period
This study was conducted among four districts (Sylhet, Habiganj, Moulvibazar and Sunamganj) at Sylhet division of Bangladesh. Sylhet division is located in North-East part of Bangladesh and between 24°30’ North latitude and 91°40’ East longitudes. The division has an area of 3490.40 square kilometers. More than three quarter of the division consists of mostly tea garden, hilly, water logged and low lying areas. The average maximum and minimum temperatures are 38°C and 10°C, respectively. The annual average rainfall is 3334 mm and humidity is 70%. The study was carried out in the Laboratory under the Department of Medicine, Faculty of Veterinary, Animal and Biomedical Science, Sylhet Agricultural University (SAU) from September 2016 to August 2017.
Collection of Fecal Samples
Freshly voided feces and/or directly from rectum were randomly collected from adult cattle. Each sample of 20-25gm of feces was collected into disposable plastic bottles. All fecal samples were collected in separate bottles to prevent potential cross contamination between fecal samples. The samples were clearly labeled with animals identification (age, sex, and breed), date and place of collection. After collection, the samples were kept on ice-box and bring to the laboratory for further process. Fecal samples collected in the field were kept refrigerated at 4°C until processed for the determination of parasite within 24 hours of collection.
Coproscopic Examination
In addition to gross examination of fecal samples (color, odor, consistency etc.) two different types of qualitative analyses, namely direct smear and sedimentation technique were followed to detect the parasitic eggs in fecal materials. The direct smear method for fecal examination was performed, as described (Hossain and Ali, 1998). In addition to direct smear approach, all the fecal samples were further analyzed using standard sedimentation technique to achieve maximum number of eggs (Soulsby, 1986). Eggs of paramphistomum spp. were identified by morphological characteristics as described (Soulsby, 1986).
Direct Smear Method
A drop of water placed on the center of a clean glass slide. A small amount of feces were taken from the sample with the help of a toothpick and spread out to form a thin smear. This can be done gently by drawing the course particles towards aside on the glass slide. A suitable cover slip may preferably be put over the smear and the slide then placed under the low power objective of a microscope for examination.
Standard Sedimentation Technique
Three grams of feces mixed in water with ten times to that of its volume in a beaker. Then the feces strained through the sieve. A quantity of this strain emulsified fluid was placed in a centrifuge tube to fill it. Then centrifuge at 2000 rpm for 1-2 minutes, supernatant fluid was poured off very carefully. Fresh water was added, shake very well and again centrifuge, and the supernatant fluid was poured off. In this way several washings were made for ultimate sediment (2-3 times centrifugation is sufficient for this purpose). A bit of the final sediment was taken out with a medicinal dropper and placed on a cleaned slide. Then covered with a cover glass and examined under microscope.
Table 1: Prevalence of paramphistomiasis in Sylhet division with regard to age and sex of the animals
| Factors | Variables | No. of animal examined | No. of positive animal | Prevalence | P-value | Total No. of animal examined | |
| Coprological examination | Age | < 3 years | 146 | 45 | 30.82% | 0.013 | 613 |
| 3-5 years | 350 | 126 | 36.00% | ||||
| > 5 years | 117 | 55 | 54.55% | ||||
| Sex | Female | 484 | 191 | 39.46% | 0.005 | ||
| Male | 129 | 35 | 27.13% | ||||
| Slaughter house survey | Age | 3-5 years | 160 | 145 | 90.63% | 0.787 | 215 |
| > 5 years | 55 | 51 | 92.72% | ||||
| Sex | Female | 30 | 28 | 93.33% | 0.750 | ||
| Male | 185 | 168 |
90.81% |
Table 2: Seasonal prevalence of paramphistomiasis in Sylhet division of Bangladesh
| Study parameters | Name of Seasons | No. of animal examined | No. of Affected Animals | Prevalence | P-value | Total examined Animals |
|
Coprology |
Summer | 126 | 47 | 37.30% |
0.413 |
613 |
| Rainy | 167 | 68 | 40.71% | |||
| Winter | 320 | 111 | 34.68% | |||
|
Slaughter house survey |
Summer | 113 | 99 | 87.61% |
0.153 |
215 |
| Rainy | 87 | 83 | 95.40% | |||
| Winter | 15 | 14 |
93.33% |
Pathological Examination
The rumen and reticulum from 215 slaughtered animals were collected and checked (weekly) from regional slaughter houses of Sylhet district in Bangladesh and examined to record the presence of mature or immature flukes. Briefly, the rumen and reticulum was inspected by opening of the rumen and reticulum for identification of rumen fluke. Washing and clearing of rumen content by flowing tap water. Collection of rumen flukes and were identified by their morphological characteristics as described (Soulsby, 1986; Panyarachun et al., 2010).
Statistical Analysis
The data from animal source (age, sex and area of collection) and laboratory work was firstly entered into Microsoft excel spread sheet and coded for analysis. Prevalence was analyzed by logistic regression test using SPSS 17.0 (SPSS Inc., USA). All tests of statistical significance were carried out at the p<0.05 using SPSS 17.0 (SPSS Inc., USA).
RESULT
Among 613 fecal samples from four districts (435 from Sylhet, 58 from Habiganj, 78 from Moulvibazar and 42 from Sunamganj) were microscopically examined, 226 (36.87%) of them were found to be infected with Paramphistomum spp (Table 3). In addition, 215 slaughtered animal’s rumen and reticulum were processed for visual ex
Table 3: Prevalence of paramphistomiasis in cattle of four districts in Sylhet division of Bangladesh by coproscopic examination
| Regions | No. of Animals Examined | No. of Affected Animals | No. of negative animal | Prevalence |
| Sylhet | 435 | 141 | 294 | 32.41% |
| Habiganj | 58 | 28 | 30 | 48.28% |
| Moulvibazar | 78 | 35 | 43 | 44.87% |
| Sunamganj | 42 | 22 | 20 | 52.31% |
amination, among them 196 animals were found positive estimating the prevalence of 91.16%.
Coproscopic Examinations
The prevalence of paramphistomiasis was found to be associated with age, sex, region and season as revealed by the multivariate analysis of risk factors. Among multivariable statistically significant with age factor (0.013), sex (0.005) was considered to the calculated p-value is less than 0.05 (Table 1). A total 613 randomly collected fecal samples were examined. Paramphistomiasis was observed highest in cattle aged above 5 years (54.55%) and lower in the animals aged less than 3 years (30.82%). From field samples, paramphistomum eggs were found 191 female (39.46%) and 35 male (27.13%) positive animals. The higher prevalence were observed in rainy season (40.71%) followed by summer (37.30%) and winter (34.68%). Age and sex of the animals was significantly (P<0.05) influence the prevalence of paramphistomiasis. Among the regions, the infection was more in Sunamganj (52.31%) and the lowest was estimated in Sylhet (32.41%).
Slaughter House Survey
In abattoir survey, paramphistomiasis was observed higher in cattle aged above 5 years (92.72%) of old and lower in the animals aged between 3-5 years (90.63%) (Figure 1). In the females (93.33%) it was higher than males (90.81%). The highest and lowest prevalence were observed in rainy (95.40%) and summer (87.61%) season respectively (Table 2).
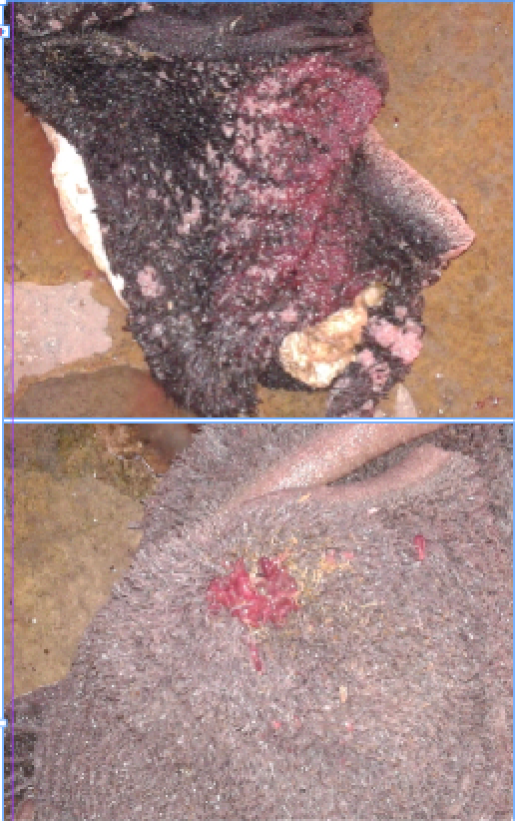
Figure 1: Paramphistomum spp. in rumen of cattle identified during visual inspection of slaughter house survey
DISCUSSION
In this study, present status of paramphistomum infection and its epidemiology were measured in cattle at Sylhet division of Bangladesh by fecal examinations and slaughter house survey. Fecal examinations revealed that the overall prevalence of paramphistomiasis in cattle was 36.87%. Paramphistomiasis thought to be a main constraint of productivity in many areas. In Asia the prevalence rate of paramphistomiasis were 30-60% still recorded (Raza et al., 2010; Sanabria, 2008). However, fecal examination of cattle and buffaloes from some limited areas of Bangladesh has also suggested that the prevalence of paramphistomiasis is very high in Bangladesh (Afazuddin, 1985; Saifuzzaman, 1996). The overall prevalence of paramphistomiasis recorded in cattle was near to similar with, Rokeya et al. (2015) who reported 30% prevalence of cattle in Banskhali upazilla, Chittagong, Bangladesh. There are several reports of higher prevalence than the present study in Bangladesh (Sarder et al., 2006; Rahman and Mondal, 1983). This variation in the prevalence may be due to agro-ecological conditions, animal husbandry practices and breeds of animal (Sarder et al., 2006). Prevalence and intensity of Paramphistomum spp. in cattle in South-Eastern Iran were measured (36.9%), which is also consistent with the present study (Khedri et al., 2015).
Highest rate of infection was found in the older cattle of more than 5 years (54.55%) which is nearly close to other investigators (Paul et al., 2011) who reported 60.3% prevalence in older cattle in Bangladesh. This result is an agreement with the earlier findings of Okafor et al. (1988) who reported that there was an age limit in the prevalence of paramphistomiasis. They also reported that heavy infection was found in cattle more than five years of age. The reason for this variation in the prevalence of infection in different age groups in cattle is difficult to explain but it might be due to an age related variation in resistance to disease and grazing habit (Okafor et al., 1988). Higher prevalence of parasitic infection in adult cattle might be due to keeping them for a longer period of time in breeding and milk production purposes or supply inadequate feed against their high demand (Sarder et al., 2006). Moreover, stress like lactation, pregnancy, nutritional deficiency which might be accounted for higher prevalence in adult cattle, Radostits et al. (1994).
The variation in the occurrence of infection in male and female cattle might be due to variation in sample size, genetic resistance of host, stress, and lack of nutrition (Samad et al., 2004; Raza et al., 2010). Paul et al. (2011) reported 59.5% prevalence of female and 45% in male. The higher percentage of infection in the females may be due to the alteration in the physiological condition of the animals during pregnancy and lactation. Higher infection in animals was found in rainy season and this may be due to the high rainfall and lodging of water which facilitates parasitic survival. Highest prevalence was found in Sunamganj (52.31%). Paramphistomum spp. mostly survives in low land and in heavy rainfall area. Sunamganj is a haor area where water staged at pasture land in maximum period of the year. An overall prevalence of paramphistomiasis was found 91.16% by slaughter house survey. This finding is nearly close to previous observators (Rolfe et al., 1991) who reported 98% paramphistomiasis. But this result is higher than others (Ayalew et al., 2016). The occurrence of paramphistomiasis in an area is influenced by a multifactorial system that is composed of hosts, parasitic agents, transmission process and environmental effects (Radostits et al., 1994). The variation in the rate of prevalence may be attributed to environmental conditions, management conditions, parasites and use of antiparamphistome drug agents. Increased incidence of Paramphistomum spp were found in adult cattle 92.72%. The finding differs with the previous reports who reported 75.2% prevalence in adults and 47.2% in young animals (Keyyu et al., 2006). The relatively high frequencies could be associated with nutritional and climatic stress, such as altitude, rainfall and temperature as well as livestock management system. In this study highest prevalence was in rainy season (95.40%) and lowest were in summer (87.61%). During the dry periods, breeding of the snails and development of the larval flukes slow down or stop completely and snails undergo a state of aestivation (FAO, 1994; Soulsby, 1986).
CONCLUSION
It may be concluded that paramphistomiasis in cattle is commonly distributed in Sylhet division of Bangladesh. It is observed that the disease is diagnosed throughout the year, but its prevalence was more in rainy season. Control of paramphistomiasis may be achieved by removal of cattle from pasture or by interrupting the life cycle of the parasite with prevention of grazing nearby water logged areas. Proper use of effective anthelmintics and strategic treatment during the dry season may reduce contamination of the snail habitat and infectivity of the pasture in the following wet season.
ACKNOWLEDGEMENT
The authors gratefully express their gratitude to Sylhet Agricultural University Research System (SAURES), Bangladesh for financial support to complete this research successfully.
CONFLICT OF INTEREST
The authors declare that there is no conflicting interest with regards to the publication of this manuscript.
REFERENCES





