Advances in Animal and Veterinary Sciences
Research Article
The Role of Sex Chromatin Figures on some Biochemical Constituents of Blood and Milk in Local She-Camels (Camelus dromedaries)
Ali J. Al-Nuaimi1, Mayada S. Hassan1, Ali M. Al-Yasari2, Yasser J. Jameel1
1College of Veterinary Medicine, University of Kerbala, Kerbala, Iraq; 2College of Veterinary Medicine, Al-Muthana University, Muthanna, Iraq.
Abstract | This study was designed to determine the roles of sex chromatin in relation to some blood and milk constitutes of she camel (Camelus dromedaries). Blood and milk samples were collected from 30 adult dairy, 5-10 years old she camel at Al Muthanna province during the period from August 2017 to February 2018.The samples were sent to the laboratory of college of veterinary medicine/ Kerbala University for analysis. The percentages of sex chromatin types including Drum stick, Sessile nodule, small club and Tear drop were 59.95%, 25.20%, 8.53%, and 6.31% respectively. The means of horizontal and vertical dimension and sex chromatin areaswere 1.150 ± 0.04 μm, 1.115 ± 0.04 μm and 0.986 ± 0.07 μm2 respectively. While, the average ± standard error of horizontal and vertical dimension and nucleus areawere 11.55 ± 0.29 μm, 11.12 ± 0.30 μm and 101.40 ± 4.76 μm2 respectively. Moreover, the sexual chromatin area / nucleus area and the number of lobes in the nucleus were 1.061 ± 0.07% and 3.82 ± 0.14 respectively. The blood glucose, total protein, total cholesterol, and total triglyceride means were118.89 ± 2.74 mg / dL, 7. 61 ± 0.18 g/dL and 70.80 ± 2.14 mg/dl and 55.28 ± 1.27 mg/dl, respectively. However, the lipid, protein, lactose and non-lipid solid materialsmeans of the milk were3.40 ± 0.11 and 3.60 ± 0.10 and 4.8 ± 0.12 and 11. 25 ± 0.17% respectively.A significant variations were appeared between different parameters of blood and milk constituents in relation to the types of sex chromatin. In conclusion, this study approved the possibility for using sex chromatin in rapid selection programs to improve the genetic properties in the local Iraqi camels population.
Keywords | Camelids, Sex chromatin, Blood, Milk
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 12, 2018; Accepted | June 27, 2018; Published | July 25, 2018
*Correspondence | Ali J Al-Nuaimi, College of Veterinary Medicine, University of Kerbala, Kerbala, Iraq; Email: saydalialneamy@yahoo.com
Citation | Al-Nuaimi AJ, Hassan MS, Al-Yasari AM, Jameel YJ (2018). The role of sex chromatin figures on some biochemical constituents of blood and milk in local she-camels (camelus dromedaries) Adv. Anim. Vet. Sci. 6(7): 306-310.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.7.306.310
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Nuaimi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Camels are among the animals that mentioned in the Quran as a God miracle (Deuraseh, 2005). Arabian camels are the important component of arid and semiarid ecosystem. Camels have economic importance for the Bedouins and also are considered as a source of milk, hid and meat (Yagil, 2000). The scientist give attention to the camel breeding, moreover they implemented a new technologies to improve the camelids breeding and its genetic characteristic and to overcome the cases of non-fertilization (Zeidan et al., 2011). Globally, there are approximately 23.9 million head according to the statistics of the World Food and Agriculture Organization. Camels belongs to Camelus species that are classified into two genus the Camelus dromedaries and Camelus bactrianus (Wardeh, 2004). Chromosomes are genetic structures within the cell nucleus that are responsible for transmitting genes from parents to offspring in the form of genes, the basic unit of one sex chromatin (Al-Issawi et al. 2013). The presence of chromatinmaterials in some body cells of females and absence it in the body cells of males, open the way for researchers to use this feature in the field genetic selection.This is called the sex chromatin or Bar bodies (Ali et al., 2008; Dyer, 2009). Review of literature revealed scarce publicationsconcerning the types of sex chromatin and its relation to blood biochemical substances in the local Iraqi camelids. Therefore, this study was designed to show the roles of different types of sex chromatin of polymorphonuclear white blood cells (neutrophil) and its relation to some biochemical features, in addition to know the best parameters that can beused as a guide for breeding selections.
Materials and methods
The study was carriedout on 30, adult dairy, 5-10 years old she camel during the period from August 2017 to February 2018, at Al-Rehab/Samawa district/Al Muthanna province. Moreover, all data and information regarding each animal were recorded. Blood samples were collected from milk vein using 10 mL vacuum tubes without and with anticoagulant (EDTA) and kept in cool box (Young, 2000). The samples were transferred immediately to the laboratory, College of Veterinary Medicine, University of Kerbala. Then, 8 blood smears were prepared from each blood sample (totally 240 blood smears). All blood smears were stained with freshly prepared Giemsa stain after fixation with alcohol. After ten minutes the slides were washed with water and air-dried. Drops of Canada balsam were placed on each slide to fix the covered slides (Coles, 1986). All prepared blood smears were examined under light microscopy using oil immersion lens (X100) magnification lens multiply by (X10) optical lens. Serum samples were collected from blood samples after centrifugation for 5 minutes (Centrifuge -T-30 Germany) at 5000 RPM/minutes and kept at -5 ° C until analysis. Serumsamples were analyzed to estimate metabolic material (glucose, total protein, cholesterol and triglycerides) using appropriate commercial kits and measured by Spectrophotometer (PD303- Germany) using different wavelength. Glucose and cholesterol; total protein and triglyceride were measured using Agappe Kit (USA) (Tietz, 1999), Spinreact Kit (Spain) (Young, 1995) and Cromatest Kit (Spain) (Young, 2000) respectively, using wavelength 505 and 500 nm; 540 nm and 500 nm respectively.
Statistical Analysis
Statistical analysis was done using SAS program, specifically the general linear method to study the effect of sex chromatin types in some biochemical constitutions (SAS, 2010). However, the chi-square test was used to compare the relative distribution of the different types of sex chromatin in the studied traits.
Results and discussion
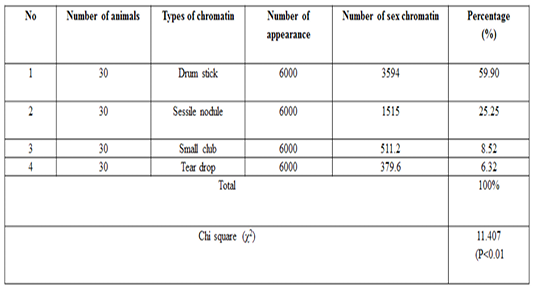
The number of sex chromatin andits distribution percentages in neutrophil (polymorphonuclear WBCs) in blood samples of she camels are presented in Table 1. Different typeof sex chromatin were seen as Drum stick (Figure 1), Sessile nodule (Figure 2), small club (Figure 3) and Tear drop (Figure 4).
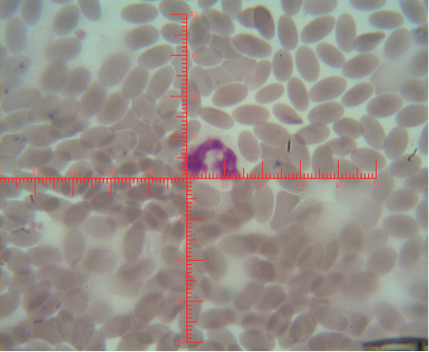
Figure 1: Shows sex chromatin types; small club, the horizontal and vertical dimension measurements in the nucleus of the neutrophils (x1000)
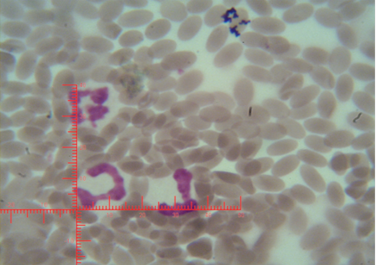
Figure 2: Shows sex chromatin type Sessile nodule, the horizontal and vertical dimension measurements in the nucleus of the neutrophils (x1000)
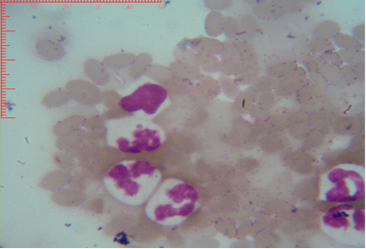
Figure 3: Shows sex chromatin type tears drop, the horizontal and vertical dimension measurements in the nucleus of the neutrophils (x1000)
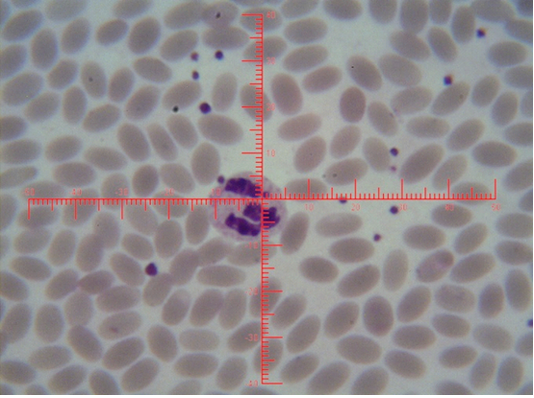
Figure 4: Shows sex chromatin type tears drop, the horizontal and vertical dimension measurements in the nucleus of the neutrophils (x1000)
A high significant (p<0.01) result was observed for the drumsticktype in compare to the other types of sex chrom-atin. The percentages of sex chromatin types including, Drum stick, Sessile nodule, small club and Tear drop were 59.95%, 25.20%, 8.53%, and 6.31% respectively. This results are compatible with previous results reported in cows (Al-Janabi and Al-Essawi, 2010), however, this results are disagreed with previous studies on sheep (Raoof et al., 2017), who reportedthe different sex chromatin percentages as 60.22%, 23.37%, 8.54% and 7.87% for sessile nodule drum stick, small club and Tear drop respectively. Meanwhile, the results of the current study is compatible with (Al Anbari and Al Khazragi, 2012) specifically in low percentages of small club and tear drop with 1.89% and 9.25% respectively, but disagreed with (Al-Jebori, 2013) with percentages of 29.35 & 14.30% ,respectively, and (Abbas, 2016) with 52.00%, 34.7%, 12.57% and 0.72% for sessile nodule, small club, drum stick and tear drop respectively.
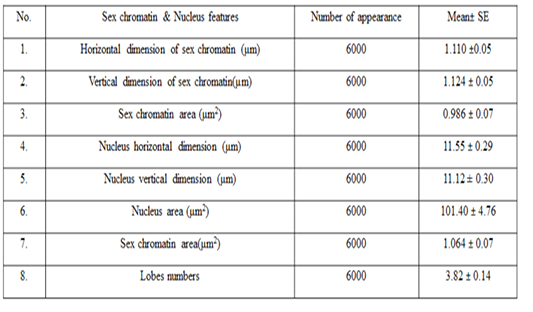
The means ± SE of sex chromatin dimensions and nucleus measurements are represented in Table 2. The horizontal and vertical dimensions and sex chromatin area were 1.110 ± 0.05 μm and 1.124 ± 0.05 μm 986.0 ± 0.07 μm2 respectively. While, the horizontal and vertical dimensions and the area of the nucleus means were 11.55 ± 0.29 μm, 11.12 ± 0.30 μm and 101.40 ± 4.76 μm2 respectively. Meanwhile, the averagepercentage of the sex chromatin area to the nucleus area was 1.064 ± 0.07%. In addition, theaverage number of lobes in the nucleus was 3.82 ± 0.14. The results of the current study are compatible with the previous study in sheep (Khazraji and Al-Nabari, 2012), who showed similar measurements 1.30 μm, 1.02 μm and 1.041 μm for horizontal dimension, the vertical dimension and sex chromatin area respectively. Meanwhile,the horizontal, vertical dimension, nucleus area and sex chromatin area / nucleus area and number of lobeswere 11.56μm, 11.33μm, 102.82μm2, 1.01% and 3.95 lobes respectively. Khazraji and Al-Nabari, (2012) showed that the horizontal and vertical length of the nucleus and nucleus area were 14.73 ± 0.29 μm, 14.36 ± 0.34 μmand 166.05 ± 5.66 μm2 respectively. While, the horizontal and vertical dimension and the area of sex chromatin were 1.096 ± 0.04 μm, 1.110 ± 0.03 μm and 986.0 ± 0.04 μm2 respectively. Moreover, the mean of sex chromatin area / nucleus area and number of lobes in the nucleus were 0.632 ± 0.04% and 4.30 ± 0.10 in cows (Al-Janabi and Al-Essawi, 2010). The results of the current study is compatible to previous study (Raoof et al., 2017) who found that the horizontal and vertical dimension, sex chromatin area and the number of lobes were 1.32 μm, 1.26 μm, 0.943 μm2 and 4.18 respectively. However, Al-Jebori, (2013) studied these features found that the horizontal and vertical dimension, the area of sex chromatin and the number of lobes were 1.02 μm, 0.761μm, 2.50μm2 and 3.43μmrespectively in female Shami goats. While, the horizontal and vertical dimension and the nucleus and sex chromatin areas / nucleus area and number of lobes were 8.12 μm, 7.76 μm, 64.73 μm2, 4.36%, and 3.43 respectively. However, the horizontal and vertical dimension and sex chromatin area were 1.02 μm, 0.96 μm and 2.37 μm respectively in local goats. Furthermore, the horizontal dimension, nucleus area and chromatin area/nucleus area and number of lobes were 7.87μm, 7.91 μm, 57.42 μm, 5.00% and 3.62 μm, respectively.
These results demonstrated that the sex chromatin area was almost constant in most farm animals, although there was a slight difference between them. And, if the slight differences were observed, they are due to differences in the size of the chromatin x (John et al., 2003; Bhatia and Shanker, 1982). Moreover, the difference in the nucleus area is also due to the difference in the size of the white blood cells (neutrophils) among animals (Khazraji and Al-Nabari, 2012).
The overall mean± SE for the tested milk samples from local Iraqi camels are presented in Table 3. The mean± SE of glucose, total protein, total cholesterol and triglycerideswere 118.89 ± 2.74 mg / dL, 7.61 ± 0.18 g / dL, 70.80 ± 2.14 mg / dl and 55.28 ± 1.27 mg / dl respectively.
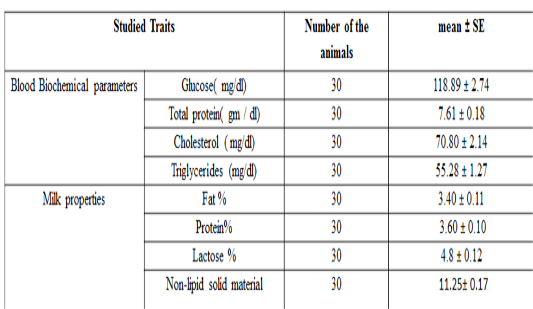
Table 3: Biochemical parameters and milk recipes studied in the local Iraqi market (general mean ± standard error)
The results of this study are compatible with previous study done on camel (Zeidan et al., 2011), who found that total protein and cholesterol concentration were 7.02 g/dl and 88.65 mg / dl respectively. Moreover, the results of the current study are also in agreement with previous researchers, who found that the total protein, glucose, cholesterol and triglyceride concentration in camels were 7.18 g / dl, 98.12, 114.23, and 36.17 mg / dL respectively (Ohri and Joshi,1961), and (6.16 g / dl), (110.04 mg / dL), (38.30 mg / dL) and, (34.30 mg /dLrespectively (Albomohsen, 2011; Ali et al., 2008). However, the results of this study are disagreed with another study in camel (Raghvendar et al., 2004). These differences in concentrations of biochemical parameters may be due to differences in animal strain, food and health status of the herd, sample size and geographic location. The mean ± standard error of fat, protein, lactose, and non-lipid solids content in milk are presented in Table 3 and were 3.46 ± 0.11, 3.52 ± 0.10, 4.14 ± 0.12 and 11.05 ± 0.17% respectively. These results are compatible with previous study (Ohri and Joshi,1961), which found that the averages of fat, protein, lactose and non-lipid content were 4, 3.46, 4.86 and 12.2% respectively. Meanwhile, the results of the current study are disagreed with (Al-Rubaie et al., 2013), who found that fat, protein, lactose, and non-lipid solids content percentage were3.13, 3.7, 4.67 and 11.29% , and also with (Raghvendar et al., 2004) with percentage of 5.5, 4.5, 5.8 and 13.95% for fat, protein and lactose respectively. These differences may be occurred due to the variations of the breed of animal, the health status of the herd and the type of nutrition and geographical location.
Acknowledgements
We would like to thank The College of Veterinary Medicine/Al Muthanna University for accepting our article in 2nd Colloquium for Camel Research and giving us this opportunity to express our science.
Conflict of interest
This research is a personal non-profit work and there is no conflict of interest.
Authors Contribution
Both of Ali J. Al-Nuaimi and Mayada S. Hassan are responsible for animal work and samples collection. Ali M. Al-Yasari and Yasser J. Jameel are responsible for data analysis, writing correction and proof reading.
References