Advances in Animal and Veterinary Sciences
Research Article
Effect of Epidural Washout with Saline on Reversing Epidural Anesthesia with Lidocaine 2% with or without Adrenaline in Dogs
Al-lethie A. Al-lethie1, Enas Elmeligy2*, Arafat Khalphallah3, Bahaa Eldeen A. Abedellaah4, Ahmed Hafez5, Usama T. Mahmoud6, Mustafa Shukry7
1Department of Surgery, Anaesthesiology and Radiology, Faculty of Veterinary Medicine, Aswan University, Aswan 81528, Egypt; 2Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt; 3Department of Animal Medicine, Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt; 4Department of Surgery, Anaesthesiology and Radiology, Faculty of Veterinary Medicine, Sohag University, Sohag 82524, Egypt; 5Department of Pharmacology, Faculty of Veterinary, Medicine, Aswan University, Aswan 81528, Egypt; 6Department of Animal Hygiene, Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt; 7Physiology department, faculty of veterinary medicine, Kafr elsheikh University, Kafr elsheikh 33511, Egypt.
Abstract | Washout of the epidural space with normal saline has been proposed to accelerate recovery from epidural block with contradictory results in human clinical studies. The present study aimed to examine the effect of epidural washout with saline on reversing epidural anesthesia with lidocaine 2% with or without adrenaline in dogs. Twenty four dogs were used in the study. Dogs were divided into four groups (n=6); group (A) epidurally injected by lidocaine 2%, group (B) epidurally injected by lidocaine 2% with adrenaline, group (C) epidurally injected by lidocaine 2% and after 30 minute received epidural washout with 10 ml normal saline and group (D) epidurally injected by lidocaine 2% with adrenaline and after 30 minute received epidural washout with 10 ml normal saline. The sensory and motor functions were evaluated and values of heart rate, respiratory rate and rectal temperature were recorded. Serum lidocaine concentrations were measured. The obtained results showed that, epidural washout with 10 ml normal saline significantly fastens the time of complete sensory and motor block recovery. There were no significant differences in heart rate, respiratory rate and body temperature. Epidural washout does not affect vascular absorption of epidural lidocaine. Epidural washout with normal saline can safely use to hasten complete sensory and motor block recovery following epidural anesthesia with lidocaine 2% with or without adrenaline in dogs.
Keywords | Epidural anesthesia, Lidocaine, Motor block, Sensory block, Washout..
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | April 07, 2018; Accepted | May 07, 2018; Published | May 31, 2018
*Correspondence | Enas Elmeligy, Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt; Email: enaselmeligy@yahoo.com
Citation | Al-Lethie AAL, Elmeligy E, Khalphallah A, Abedellaah BEA, Hafez A, Mahmoud UT, Shukry M (2018). Effect of epidural washout with saline on reversing epidural anesthesia with lidocaine 2% with or without adrenaline in dogs. Adv. Anim. Vet. Sci. 6(5): 207-212.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.5.207.212
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Al-Lethie et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
General anesthesia is the most common anesthetic protocol in small animals. The cost of general anesthesia, technical knowhow and the risk factors are some of the challenges that discourage the choice of the general anesthesia in some surgical procedures (Abubakar et al., 2015). Epidural anesthesia has been advocated as an alternative to general anesthesia for surgical procedures caudal to the diaphragm in dogs but it was superior for procedures involving the hind limbs and perineal area (Rauser et al., 2004).
There is no reversal agent for local anesthetics and the resolution of epidural anesthesia depends on the redistribution of local anesthetics from neural tissues and its elimination by systemic uptake (Park et al., 2009).
The use of short-acting local anesthetics for epidural analgesia shortens anesthetic time but it does not allow one to extend the duration of the block in the case of unexpectedly prolonged surgery (Rodrıguez et al., 2001). Conversely prolonged sensory and motor block following epidural block after short surgical procedures increase post anesthetic care unit time and expense, dissatisfaction of the animal’s owner and may cause postoperative hind limb paralysis and urinary retention (Shoeibi et al., 2007).
Washout of the epidural space at the termination of operation has been proposed to accelerate recovery from epidural block with contradictory results in human clinical studies (Rodrıguez et al., 2001; Williams et al., 2011; Attia et al., 2015; Couture et al., 2016), therefore, the current study aims to investigate whether washout of the epidural space with normal saline could provide a clinically significant faster recovery from motor and sensory blockade after epidural anesthesia by lidocaine with or without adrenaline in dogs.
MATERIAL AND METHODS
Ethics Statement
The present study was approved by The National Ethical Committee of the Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt which basically conform to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health in the USA (NIH publication No. 86-23, revised 1996).
Experimental Animals
The present study was conducted on twenty four clinically healthy adult local dogs of both sexes (twelve males and twelve non pregnant females), aged 2 to 4 years old with body weight ranged between 20- 25kg. The animals were divided into four groups each of six dogs (three males and three non-pregnant females). The animals were housed in individual cages with food and water ad libitum.
Procedures
Anesthetic technique: Food, but not water, was withheld for 12 hours before the study. Catheterization of cephalic vein for blood sampling was performed. The lumbosacral area was clipped and scrubbed with povidone iodine (10%). Lidocaine 2 % was infiltrated subcutaneously over the lumbosacral joint space. A sterile epidural needle was inserted into epidural space at L7–S1 interspace. Proper placement of the needle was determined by loss of resistance and by ease of injection. The medications were administered over approximately 60 seconds in each dog according to procedure described by (Otero and Campoy, 2013).
Drugs used: Debocaine (Lidocaine HCl 2%), Chemicals Company for El-Debeiky Pharma, Egypt. Lidocaine with adrenaline (Lidocaine HCl 2% with adrenaline 0.00227%), a commercially prepared lidocaine-adrenaline combination, Norbrook company, UK.
Anesthetic protocols: Epidural anesthesia was produced in group (A) with lidocaine 2%, 1 mL/6 Kg, in group (B) epidural anesthesia was produced by lidocaine 2% with adrenaline 0.00227%, 1 mL/6 Kg, in group (C) epidural anesthesia was produced by lidocaine 2%, 1 mL/6 Kg and after 30 minute the dogs received epidural washout with 10 ml normal saline and in group (D) epidural anesthesia was produced by lidocaine 2% with adrenaline 0.00227%, 1 mL/6 Kg and after 30 minute the dogs received epidural washout with 10 ml normal saline.
Local anesthetic evaluation: Sensory and motor block were evaluated according to Dehghani and Sadegh (2007).
Sensory blockade: Sensory blockade (analgesia) assessed by response to the pressure of haemostat forceps applied to the skin of the hind limbs. Cranial extension of analgesia also recorded by lack of a response to hemostat pressure applied first in the perineal area and then moved cranially toward the thoracic region. Loss of pain response verified by the absence of movement, groaning, biting attempts, looking at the limb, and head shaking after the painful stimulus was performed with the hemostats. Responses measured each minute until no reaction occurred and then at 5 minutes intervals until a response occurred.
Onset of analgesia calculated as the time interval (In minutes) between the epidural drug injection and loss of pain response. Duration of analgesia calculated as the time interval (in minutes) between the loss and return of pain response.
Motor blockade: Onset of motor blockade calculated as the time interval (in minutes) between the epidural drug injection and loss of weight support. Duration of motor blockade calculated as the time interval (in minutes) between loss of weight support and the dogs’ ability to stand.
Physiological vital parameters: Heart rate (HR), respiratory rate (RR) and rectal temperature (RT) were recorded for each dog before epidural injection (time 0) and 15, 30, 60, 90 and 120 minutes after epidural injection. Heart rate was determined with stethoscope, respiratory rate was determined by visual observation of chest excursions and rectal temperature was measured using a clinical thermometer.
Vascular absorption: Venous blood samples were collected through indwelling cephalic vein catheter in sterile glass tubes containing no anticoagulant for lidocaine concentration measurement at 15, 30, 45, 60, 90, 105 and 120 minutes after epidural injection. Within few hours of blood collection, serum separated by centrifugation. After centri-
Table 1: Local anesthetic effect
| Loss of weight support (min) | Onset of analgesia (min) | Duration of analgesia (min) | Duration of recumbency (min) | |
| Group A |
2.20±0.13b |
6.47±0.43b |
97.00±3.31a |
116.67±3.69a |
| Group B |
3.70±0.15a |
7.67±0.52a |
103.17±4.94a |
130.17±4.49a |
| Group C |
2.55±0.25b |
6.65±0.42b |
66.50±2.05c |
76.50±2.38c |
| Group D |
3.63±0.16a |
7.98±0.33a |
81.33±1.76b |
92.67±3.15b |
| P value | 0.000 | 0.054 | 0.000 |
0.000 |
Different superscripts within the same rows depict significant differences among groups at p < 0.05.
Table 2: Effect on heart rates (beats/min)
| 0 Time | 15 min | 30 min | 60 min | 90 min | 120 min | |
| Group A | 94.00±5.07 | 96.00±5.37 | 97.17±5.06 | 93.67±3.95 | 92.33±4.40 | 94.33±5.34 |
| Group B | 86.83±4.62 | 88.33±4.79 | 84.83±4.60 | 86.33±4.74 | 85.67±3.98 | 84.17±3.45 |
| Group C | 88.17±5.26 | 83.50±5.18 | 84.50±4.29 | 84.83±4.03 | 88.67±4.27 | 88.17±3.20 |
| Group D | 88.50±5.20 | 86.33±4.95 | 83.67±4.29 | 84.33±4.14 | 87.83±2.61 | 88.17±3.19 |
| P value | 0.759 | 0.368 | 0.149 | 0.391 | 0.679 |
0.350 |
Different superscripts within the same rows depict significant differences among groups at p < 0.05.
Table 3: Effect on respiratory rates (breaths/min)
| 0 Time | 15 min | 30 min | 60 min | 90 min | 120 min | |
| Group A | 23.33±1.56 | 24.00±1.75 | 23.67±1.98 | 23.00±1.75 | 22.67±1.86 | 23.00±1.88 |
| Group B | 24.67±2.60 | 24.33±1.65 | 24.00±1.51 | 24.50±1.84 | 23.50±2.05 | 25.00±2.03 |
| Group C | 23.00±2.08 | 24.67±1.73 | 23.17±1.74 | 24.00±2.29 | 23.83±2.04 | 23.67±1.45 |
| Group D | 24.00±2.42 | 23.67±1.67 | 24.17±1.74 | 24.50±1.57 | 23.00±2.10 | 24.00±1.88 |
| P value | 0.952 | 0.978 | 0.978 | 0.934 | 0.977 |
0.889 |
Different superscripts within the same rows depict significant differences among groups at p < 0.05.
Table 4: Effect on body temperature (ºC)
| 0 Time | 15 min | 30 min | 60 min | 90 min | 120 min | |
| Group A | 38.97±0.11 | 38.85±0.17 | 38.77±0.13 | 38.92±0.11 | 38.97±0.10 | 39.12±0.11 |
| Group B | 39.05±0.12 | 39.02±0.16 | 38.95±0.15 | 38.90±0.15 | 38.97±0.16 | 38.92±0.11 |
| Group | 38.97±0.09 | 38.85±0.13 | 39.07±0.09 | 39.00±0.13 | 38.93±0.16 | 38.95±0.15 |
| Group | 38.93±0.09 | 38.97±0.15 | 38.87±0.16 | 38.92±0.15 | 38.88±0.12 | 39.02±0.10 |
| P value | 0.872 | 0.815 | 0.466 | 0.954 | 0.968 |
0.667 |
Different superscripts within the same rows depict significant differences among groups at p < 0.05.
trifugation, a minimum of 1 ml of serum was stored in a polypropylene vial and frozen at –80°C until assayed. The serum concentration of lidocaine was determined by Ultra Performance Liquid Chromatography (UPLC) with solid phase extraction and UV detection, Waters Company, Singapore (Al Nebaihi et al., 2017).
Statistical Analysis
The values were expressed as the mean ± SE. All data were analyzed using one way analysis of variances (ANOVA) followed by Tukey’s test using SPSS 16.0 statistical software (Spss, Inc, Chicago, IL).
RESULTS
Epidural anesthesia was produced in all dogs following administration of lidocaine or lidocaine with adrenaline (Table 1). Times to onset of sensory and motor block were significantly less in dogs receiving epidural lidocaine (group A and C) than dogs receiving epidural lidocaine with adrenaline (group B and D).
Durations of sensory and motor blockade were significantly less in dogs receiving epidural washout with 10 ml normal saline (group C and D) than control groups (group A and B).
Durations of sensory and motor blockade were significantly less in dogs received epidural washout with 10 ml normal saline after epidural injection with lidocaine (group C) than dogs received epidural washout with 10 ml normal saline after epidural injection with lidocaine with adrenaline (group D).
After washout the epidural space, the regression of motor block is faster than the regression of sensory block and this is more obvious in dogs received epidural washout with10 ml normal saline after epidurally injection with lidocaine with adrenaline (group D) than dogs received epidural washout with 10 ml normal saline after epidurally injection with plain lidocaine (group C).
Cutaneous analgesia ranged from coccygeal vertebrae to approximately L1 and was similar in spread on both sides of the spine in both control and experimental groups.
Vascular Absorption
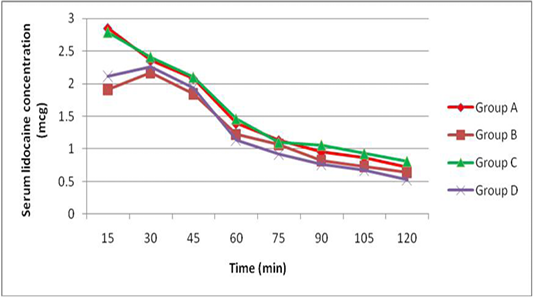
The peak serum concentration (Cmax) was achieved 15 min after lidocaine administration (group A and C) and was achieved 30 min after administration of lidocaine with adrenaline (group B and D). The mean serum concentrations of lidocaine were higher in dogs receiving epidural lidocaine (group A and C) than dogs receiving epidural lidocaine with adrenaline (group B and D) at all times after administration. There was no significant differences in the mean serum concentrations of lidocaine after administration of epidural washout (Figure 1).
Statistical Analysis
It revealed that there were no significant differences in heart rate, respiratory rate and body temperature in comparison to the base line value in all groups during the study (Table 2, 3 and 4).
DISCUSSION
Epidural anesthesia is one of the most common regional anesthetic techniques in veterinary practice. It creates by injection of the local anesthetic within the epidural space leading to blocking sensory and motor nerves (Vnuk et al., 2006; Steagall et al., 2017). Lidocaine is commonly used local anesthetic for epidural anesthesia. It has a rapid onset and moderate duration of action (Khan et al., 2015), however, addition of adrenaline to it decreases local blood flow, slowing the systemic absorption of local anesthetic agent, which prolong the anesthetic duration (Kayode, 2017).
There is no reversal agent for local anesthetics and the resolution of epidural anesthesia depends on the redistribution of local anesthetics from neural tissues and its elimination by systemic uptake (Park et al., 2009). Washout of the epidural space with saline has been proposed to accelerate recovery from epidural block with contradictory results in human clinical studies (Rodrıguez et al., 2001; Williams et al., 2011; Attia et al., 2015; Couture et al., 2016). However, the anatomical dimensions of the respective epidural spaces, the differences in vascularity, the amount of epidural fat, and the number and type of nerves present at the level of the cauda equina may affect both the pharmacokinetic and pharmacodynamic of an epidurally administered drug formulation (Doherty et al., 1996).
Our study showed that washout the epidural space with 10 ml normal saline 30 minutes after induction of epidural anesthesia hasten the recovery of motor and sensory functions either by using 2% lidocaine with or without adrenalin in dogs similar to human studies using plain lidocaine (Shoeibi et al., 2007) or lidocaine with adrenaline (Sitzman et al., 2001). Other studies found that such washout hastened motor recovery alone (Johnson et al., 1990; Williams et al., 2011) or hastened sensation recovery alone (Rodriguez et al., 2001). On the other hand, Couture et al. (2016) found no significant time difference between full motor and sensory recovery, furthermore, they denied the clinical significance of such technique.
Exactly how normal saline injections hasten return of sensory and motor function after epidural anesthesia remains inconclusive. A proposed mechanism of action is that injection of normal saline solution into the epidural space results in rostral and caudal spread of the dilute lidocaine solution within the epidural space could result in the exposure of lidocaine to large venous and lymphatic surface area and hence greater vascular uptake (Williams et al., 2011). Saline injection into the epidural space appears to augment both secretion and clearance of CSFand may therefore enhance elimination of local anesthetic from the subarachnoid space (Cousins and Bridenbaugh, 2009).
Our study failed to demonstrate these speculations because there is no difference in the serum lidocaine concentrations before and after washout (Figure 1) and this constituted with (Chan et al., 1999) who found similar serum lidocaine concentrations following epidural washout using 1, 20, and 40 ml of saline and confirmed that vascular absorption of epidural lidocaine is unaffected by epidural washout. Furthermore there was no sensory level progression after epidural washout in this study and other study by Shoeibi et al. (2007).
Epidural washout with normal saline may dilute residual unbound local anesthetic in the epidural space, thereby quickly decreasing local anesthetic concentration and reversing the concentration gradient required to penetrate the neural tissues (Chan et al., 1999) and this agree with in vitro washing rat sciatic nerve preparations with ringer solution reverses bupivacaine- induced neural blockade in approximately 25 min (Gissen et al., 1980).
In our study, epidural washout with saline following epidural anaesthesia, hasten regression of motor block faster than sensory block and this may attribute to the dilution of the anesthetic with saline and minimizing of its concentration at the neural tissues, which expected to block the sensory nerves more effectively than motor ones which need higher concentration and resulting in longer sensory block regression time (Park et al., 2009; Nishiyama, 2012), this is because the comparatively larger diameter and the more myelination in motor nerve fibers (Ford et al., 1984).Furthermore the delay in sensory regression was more obvious in (group D) because the addition of vasoconstrictor adrenaline to lidocaine has been reported to decreases local blood flow, slowing the systemic absorption of diluted lidocaine which improve the duration of the sensory blocks (Kayode, 2017).
In the present study we make flushing only with fixed volume of 10 ml and we did not observe any complication and this agree with other studies which supporting the safety of epidural washout in enhancing recovery from sensory and motor block following epidural anesthesia (Shoeibi et al., 2007; Park et al., 2009) and this in contrast with (Rodriguez et al., 2001) who found several side effects associated with washing the epidural space with large volume of saline, such as increased CSF pressure, intracranial hypertension, paraspinal muscle spasm, acute back pain, lower extremity radicular pain, headache, and temporary visual deficits may occur without additional reduction in the recovery times.
Statistical analysis revealed no differences in heart rate, respiratory rate and body temperature in comparison to the base line value in all groups during the study. These vital parameters do not change significantly after epidural administration of lidocaine with or without adrenaline in dogs (Vnuk et al., 2011) or after washout with normal saline (Attia et al., 2015).
CONCLUSION
Epidural washout with normal saline can be safely used to hasten complete sensory and motor block recovery following epidural anesthesia with lidocaine 2% with or without adrenaline.
AUTHORS’ CONTRIBUTION
All authors conducted the study equally, analyzed and discussed the results, wrote and approved the final manuscript.
CONFLICT OF INTERESTS
There is no conflicting interest with regards to the publication of this research work.
REFERENCES