Advances in Animal and Veterinary Sciences
Research Article
Hemato-biochemical Parameters as Comparative Tools and Prognostic Indicators in Urine Retention Cases with Intact or Ruptured Urinary Bladder in Buffalo Calves
Hager Tarek H. Ismail
Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University 1 Alzeraa Street, Zagazig City, Sharkia Province, Egypt, Postal Code 44511.
Abstract | The aim of this study was to compare the health status and prognosis of buffalo calves with retention of urine either with intact or ruptured bladder by studying the panel of biochemistry and hematology variables. Blood specimens were collected from male buffalo calves (n=30), which were diagnosed with urine retention either with intact or ruptured urinary bladder on the basis of clinical signs and ultrasonogarphic examination besides control animals (n=15) to perform the different biochemical and hematological tests. Anorexia, depression and abdominal distension were the obvious clinical signs in the affected calves. Ultrasonographically, an anechoic distended structure representing the urinary bladder revealed urine retention with an intact bladder while the presence of free anechoic fluid in the abdominal cavity indicates urine retention with ruptured bladder. Laboratory findings in both the affected groups revealed an increase in serum creatinine, urea and bilirubin (total, direct and indirect) concentrations and aspartate aminotransferase and alkaline phosphatase activities, hyponatremia, hypochloremia, hypocalcemia, hyperphosphatemia, hypomagnesemia, hyperproteinemia, hypoalbuminemia, hyperglobulinemia,relative polycythemia, leukopenia, neutrophilia, lymphopenia, monocytosis and eosinophilia in comparison to the control group, almost of these analytes were changed sharply in ruptured urinary bladder cases in comparison with intact urinary bladder cases. Hyperkalemia was observed in the ruptured bladder group only. In conclusion, buffalo calves which suffered from urine retention with ruptured bladder have severe alterations in most of biochemical and hematological analytes in comparison with the intact bladder group and some of these cases may die from severe uraemia and electrolytes disturbance (poor prognosis).
Keywords | Biochemical, Buffalo calves, Hematological, Prognosis, Urine retention.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 06, 2018; Accepted | March 16, 2018; Published | April 11, 2018
*Correspondence | Hager Tarek H Ismail, Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University 1 Alzeraa Street, Zagazig City, Sharkia Province, Egypt, Postal Code 44511; Email: hager_vet@hotmail.com
Citation | Ismail HTH (2018). Hemato-biochemical parameters as comparative tools and prognostic indicators in urine retention cases with intact or ruptured urinary bladder in buffalo calves. Adv. Anim. Vet. Sci. 6(4): 148-155.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.4.148.155
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Ismail. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Buffaloes are economically important livestock species consider as a source of meat and milk and valuable work animals, especially in low-income countries (Arefaine and Kashwa, 2015). One of the most important health problems in buffalo’s species is urine retention, which may occurs as consequence to urolithiasis and leads to high economic losses (Kumar et al., 2011; Abu-Seida et al., 2012). Occurrence of urolithiasis related mainly to a complex of various predisposing factors such as seasonal influence where urolithiasis reaches peak in winter season (Radostits et al., 2000; Radostits et al., 2007). Also, changes in given diet from milk to concentrate after weaning consider as a main factor for development of obstructive urolithiasis in young ruminants (Sharma et al., 2007; Rafee et al., 2015). In addition to the anatomy of the male ruminant urinary tract where long convoluted sigmoid flexure is a common site for a lodge of calculi in ruminant species (Khan et al., 2007).
According to the severity of urine flow obstruction and the reaction of surrounding tissue different pictures of clinical signs can be observed. Obstruction of the urinary tract by calculi may be either complete or incomplete. Complete obstruction results in several degrees of exaggerated and prolonged urination posture, urine dribbling, and hematuria. Pressure inside urinary bladder increases by prolongation of obstruction time (Biswas and Saifuddin, 2015; Gazi et al., 2015). Clinical diagnosis of urine retention depends mainly on the case history, clinical signs, physical examination, and ultrasonography which considers as a confirmatory tool for diagnosis in these cases (Makhdoomi and Gazi, 2013).
Systemic disturbances may qualitatively similarly, but quantitatively may be differ between cases of urine retention either with intact or ruptured bladder which may be detected by laboratory tests. Thus, the present study was conducted to compare the health status of buffalo calves cases which suffered from urine retention either with intact or ruptured bladder by studying the panel of biochemistry and hematology variables to confirm the possible prognosis.
MATERIALS AND METHODS
Animals
The present study was carried out on thirty male buffalo calves (Bubalus bubalis) (aged 3-6 months), brought with the history of urine retention to the Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Zagazig University, Egypt. At the time of cases arrival to the hospital, complete history regarding (age, feeding and duration of retention) was taken, preliminary general and clinical examinations were performed. Fifteen apparently healthy buffalo calves were used in this study as a control group. The study protocol was carried out in accordance with the Egyptian laws and university guidelines for animals care and approved by the Ethics Committee of the Faculty of Veterinary Medicine, Zagazig University, Egypt.
Grouping
Group A healthy buffalo calves used as control, obtained from the same diseased animals farms.
At the time of animal’s admission to hospital, on the basis of clinical signs and ultrasonographic examination of the urinary system, the buffalo calves with urine retention continued to (3±1 days) were classified into:
Group B buffalo calves with urine retention and had intact urinary bladder (distended) (n=15).
Group C buffalo calves with urine retention and had ruptured urinary bladder (n=15).
Ultrasonographic Examination
Transabdominal ultrasonography of the urinary bladder was done on standing position by using of a real-time B-mode-Sonoscape® machine-(China), 3.5/ 5MHz transabdominal transducer.
Sampling
Blood specimens were collected from buffalo calves by jugular vein puncture at the time of admission to the hospital. The first portion (5 ml) was collected without anticoagulant in a sterile test tube for separation of serum for biochemical analysis. The second portion (1 ml) was transferred into dipotassium ethylenediaminetetraacetic acid (K2-EDTA) coated tubes for hematological analysis. All procedures in this study were performed under informed consent of the owners.
Biochemical Analysis
Serum was used to estimate creatinine, urea, potassium, sodium, chloride, calcium, phosphorus, magnesium, total proteins and albumin concentrations, aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activities and bilirubin (total and direct) concentration. All of these analytes were estimated by using commercial diagnostic kits purchased from Diamond Diagnostic Company, Spinreact, Vitro ,ELITech and Roche Diagnostics by using of photometer 5010 (Robert Riele GmbH and co-kg, Germany), Hitachi 902 auto analyzer (Roche Diagnostics, Auckland City, Germany) and electrolyte analyzer (EasyLyte, Medica, USA). Serum globulins and indirect bilirubin concentrations were calculated mathematically by subtracting albumin from total proteins and direct bilirubin from total bilirubin, respectively.
Hematological Analysis
The complete blood count was estimated by using an automated blood cell analyzer (Sysmex XT-2000iV, Kobe, Japan).
Statistical Analysis
Data were analyzed using one-way analysis of variance (ANOVA), Tukey’s HSD multiple comparison tests was used to test the significance differences between the mean values. Variability in the data was expressed as the pooled SEM and the alpha level for determination of significance was 0.05. Means in the same row followed by different letters were significantly different and the highest value was represented by the letter (a).
RESULTS
Clinical Observations
Regarding the results of general and clinical examination of the affected cases, intact urinary bladder group showed symptoms of restlessness, anorexia, abdominal pain, kicking at the belly, frequent unsuccessful trial of urination with dribbling of urine, straining, tail switching and grating of the teeth. On the other hand, ruptured urinary bladder group showed dullness, anorexia, variable degrees of dehydration, tachycardia, polypnea, no colic pain with abdominal distension.
Ultrasonographic Findings
Transabdominal ultrasonogarphic examination was used to reveal the status of bladder (ruptured/ intact).Intact bladder was observed as anechoic distended round structure representing the urinary bladder with tensed wall in buffalo calf with urine retention and intact urinary bladder (Figure 1).While ruptured bladder was seen as free anechoic fluid in the abdominal cavity which representing urine from a ruptured bladder with the presence of hyperechoic floating substance (arrow) representing fibro gelatinous material results from the reaction of the urine with the peritoneal cavity (Figure 2).
Some Kidney Function Tests and Serum Electrolyte Panel
According to the results in Table (1), A highly significant increase in serum creatinine, urea and phosphorus concentrations was observed in both groups in comparison to the control group, however, the greatest increase was observed in ruptured urinary bladder group. Hyperkalemia was observed in ruptured urinary bladder group in comparison to the control group, while a non significant change was observed in intact bladder cases. Hyponatremia, hypochloremia, hypocalcemia and hypomagnesemia were observed in both groups in comparison to the control group, however, the lowest values of serum sodium and calcium observed in ruptured and intact urinary bladder cases, respectively.
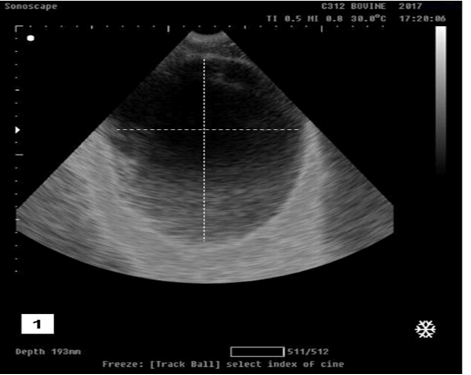
Figure 1: Ultrasonographic image showing anechoic distended round structure representing the urinary bladder with tensed wall in buffalo calf with urine retention and had intact urinary bladder.
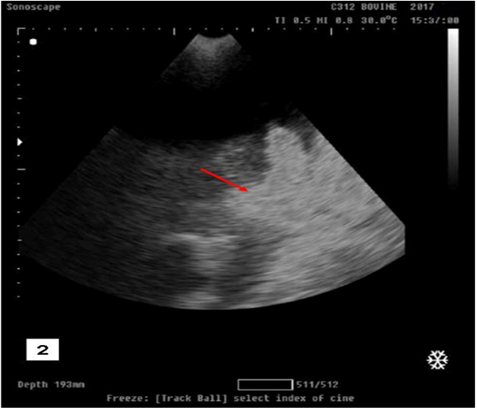
Figure 2: Ultrasonographic image showing free anechoic fluid in the abdominal cavity which representing urine from a ruptured bladder with the presence of hyperechoic floating substance (arrow) representing fibro gelatinous material results from the reaction of the urine with the peritoneal cavity in buffalo calf with urine retention and had ruptured urinary bladder.
Table 1: Some kidney function tests and serum electrolyte panel in healthy and affected buffalo calves.
| Variables | Experimental groups | |||||
| Gp.(A) | Gp.(B) | Gp.(C) | SEM1 | p- value | ||
| Creatinine (mg/dl) |
1.50c |
3.01b |
8.83a |
0.84 | 0.000 | |
| Urea (mg/dl) |
55.52c |
75.56b |
199.48a |
17.04 | 0.000 | |
| Potassium (mmol/l) |
5.68b |
5.83b |
6.67a |
0.12 | 0.000 | |
| Sodium (mmol/l) |
149.30a |
139.16b |
130.73c |
2.15 | 0.000 | |
| Chloride (mmol/l) |
111.28a |
83.62b |
82.66b |
3.55 | 0.000 | |
|
Calcium (mg/dl) |
11.23a |
8.15c |
8.44b |
0.37 | 0.000 | |
| Phosphorus (mg/dl) |
5.25c |
7.14b |
9.28a |
0.44 | 0.000 | |
| Magnesium (mg/dl) |
1.88a |
1.60b |
1.59b |
0.03 |
0.000 |
|
1SEM: Standard error of the mean
Means bearing different superscripts within the same row are significantly different (P<0.05).
Gp. (A) Control group, Gp. (B) Intact urinary bladder group, Gp. (C) Ruptured urinary bladder group.
Proteinogram and some Liver Functions Tests
Summarizing the results of Table (2), hyperproteinemia and hyperglobulinemia were observed in both groups in comparison to the control group, however, the highest values of these analytes were observed in intact urinary bladder group. Hypoalbuminemia was observed in both groups in comparison to the control group, however, the lowest value was observed in intact urinary bladder group. Serum AST activity showed highly significant increase in both groups in comparison to the control group, however, the highest value was observed in ruptured urinary bladder group. Serum ALP activity and bilirubin (total, direct and indirect) concentration showed highly significant increase in both groups in comparison to the control group, however, the highest values of these analytes were observed in intact urinary bladder group.
Hematological Findings
Data in Table (3) revealed a highly significant increase in red blood corpuscles (RBCs) count, packed cell volume (PCV) value and hemoglobin concentration (Hb) in both groups in comparison to the control group, however, the highest values of RBCs count and PCV value were observed in intact urinary bladder group, while the highest value of Hb concentration was observed in ruptured urinary bladder group. Leukopenia and lymphopenia were observed in both groups in comparison to the control group, however, the lowest value of the total leukocytes count was observed in ruptured urinary bladder group while the lowest value
Table 2: Proteinogram and some liver functions tests in healthy and affected buffalo calves.
| Variables | Experimental groups | ||||||
| Gp.(A) | Gp.(B) | Gp.(C) | SEM1 | p- value | |||
| Total Proteins (g ⁄dl) |
5.79c |
6.61a |
6.48b |
0.09 | 0.000 | ||
|
Albumin (g ⁄dl) |
3.51a |
2.52c |
3.14b |
0.11 | 0.000 | ||
|
Globulins (g ⁄dl) |
2.28c |
4.09a |
3.34b |
0.20 | 0.000 | ||
| AST (U/L) |
110.20c |
161.04b |
239.40a |
14.21 | 0.000 | ||
| ALP (U/L) |
112c |
170.40a |
127.60b |
6.61 | 0.000 | ||
|
Total bilirubin (mg/dl) |
0.67c |
1.57a |
0.81b |
0.10 | 0.000 | ||
|
Direct bilirubin (mg/dl) |
0.15c |
0.27a |
0.22b |
0.01 | 0.000 | ||
|
Indirect bilirubin (mg/dl) |
0.52c |
1.30a |
0.59b |
0.09 | 0.000 | ||
1SEM: Standard error of the mean
Means bearing different superscripts within the same row are significantly different (P<0.05).
Gp. (A) Control group, Gp. (B) Intact urinary bladder group , Gp. (C) Ruptured urinary bladder group, AST=Aspartate aminotransferase, ALP=Alkaline phosphatase
Table 3: Hematological findings in healthy and affected buffalo calves.
| Variables | Experimental groups | ||||
| Gp.(A) | Gp.(B) | Gp.(C) | SEM1 | p-value | |
|
RBCs (×106⁄µl) |
6.41c |
8.89a |
8.10b |
0.27 | 0.000 |
| PVC (%) |
29.26c |
42.10a |
37.90b |
0.24 | 0.000 |
| Hb (g%) |
10.22c |
11.81b |
12.38a |
1.44 | 0.000 |
|
T.L.C(×103/µl) |
11.74a |
10.59b |
10.10c |
0.18 | 0.000 |
|
Neutrophils (×103/µl) |
3.25c |
6.90a |
4.96b |
0.40 | 0.000 |
|
Lymphocytes (×103/µl) |
8.11a |
2.94c |
4.33b |
0.58 | 0.000 |
|
Monocytes (×103/µl) |
0.25c |
0.51a |
0.44b |
0.02 | 0.000 |
|
Eosinophils (×103/µl) |
0.13c |
0.24b |
0.37a |
0.02 |
0.000 |
1SEM: Standard error of the mean
Means bearing different superscripts within the same row are significantly different (P<0.05).
Gp. (A) Control group, Gp. (B) Intact urinary bladder group, Gp. (C) Ruptured urinary bladder group, RBCs=Red blood corpuscles, Hb=Hemoglobin, PCV=Packed cell volume, T.L.C.=Total leukocytic count
of lymphocytes count was detected in intact urinary bladder group. Neutrophilia, monocytosis and eosinophilia were observed in both groups in comparison to the control group, however, the highest values of these analytes were observed in intact urinary bladder group except eosinophils count where the highest value was observed in ruptured urinary bladder group.
DISCUSSION
Combining the clinical observations with ultrasonographic findings in urine retention cases contributed as primary diagnosis tool during arrival of cases to clinic. The pain and colic were the main signs in the cases with intact bladder may be due to complete obstruction of urine flow and severe pressure on bladder wall (Biswas and Saifuddin, 2015). While the main observations in ruptured bladder cases were the distension of the abdomen without colic or pain reaction may be due to rupture of urinary bladder, which decreased pressure on the urinary bladder wall and subsequently relief pain reaction, accumulation of urine from ruptured bladder lead to abdominal distension (Rafee et al., 2015)
In the present study, highly significant increase in serum creatinine concentration was observed in both the affected groups in comparison to the control group might be due to increased rate of creatinine reabsorption from prolonged stagnant urine in the intact urinary bladder beside renal insufficiency due to urine flow back and accumulation inside the kidneys (hydronephrosis) (Singh, 2005). Serum creatinine concentration in ruptured urinary bladder cases was observed in a higher degree in comparison to intact bladder cases could be due to movement of creatinine after rupture of the bladder and leakage of urine from the peritoneal fluid to the blood but with slow rate in comparing with urea as it has a larger molecular size (Donecker and Bellamy, 1982).
Accumulation of urine in the urinary bladder for a long time more than the normal period in urine flow obstruction cases leads to more reabsorbing of urea from the stagnant urine into the blood circulation and causes uraemia. Also, urine back pressure towards the kidney reduces the renal function in urine production by reducing the glomerular filtration rate and ultimately reduces urea excretion in urine and accumulates in the blood stream (Sharma et al., 2006). This mechanism explains the reason behind the raised values of serum urea in the cases of urine retention. Serum urea concentration in ruptured urinary bladder cases was observed in a higher degree in comparison with intact bladder cases could be due to movement of urea from the high concentration in the peritoneal cavity to the interstitial and intravascular compartments (Smith, 2002).
During this study, the occurrence of hyperkalemia in ruptured urinary bladder cases may be due to the movement of potassium from peritoneal cavity where urine has a higher concentration of potassium to circulating fluid according to concentration gradients law (Donecker and Bellamy, 1982). Hyponatremia and hypochloremia were observed in both the affected groups in comparison to the control group may be due to impairment of tubular reabsorption of sodium and chloride. Abnormalities in function of renal tubules are common in cases of urinary tract obstruction (Klahr et al., 1986). Hyponatremia was more obvious in urinary bladder rupture cases in comparison with intact bladder cases could be due to invading of peritoneal cavity with the urine after bladder rupture which has a low concentrations of sodium so these ions move from interstitial compartment into the peritoneal cavity which lead to depression of serum concentration of it (Donecker and Bellamy, 1982; Sockett et al., 1986).
Hypocalcemia and hyperphosphatemia observed in both the affected groups in comparison to the control group may be due to renal insufficiency progress as the result of hydronephrosis and urine back pressure with decreasing of glomerular filtration rate and subsequent phosphorus retention and development of hyperphosphatemia. In this case, hypocalcemia occurs as a direct physicochemical effect of hyperphosphatemia in beginning of renal dysfunction then after progression of the disease condition, hypocalcaemia may occur due to other reasons such as impaired intestinal calcium absorption due to lack of renal hydroxylation of 25-hydroxycholecalciferol (Kaneko et al., 1997). Hyperphosphatemia in ruptured urinary bladder cases occurred with a higher degree in comparison to intact bladder cases could be due to the high rate of phosphorous absorption from peritoneum, which filled with the urine to the blood circulation (Varley, 1988).
Hypomagnesemia observed in both the affected groups in comparison to the control group could be due to impairment of the tubular reabsorption of magnesium after the occurrence of renal obstruction. Also, impairment of tubular sodium reabsorption after the occurrence of obstruction lead to hypomagnesemia as magnesium transport passively follows of sodium (Shafik and Dirks, 1992; Taal et al., 2012).
Hyperproteinemia observed in both the affected groups in comparison to the control group may be due to concurrent hyperglobulinemia which occurred as a result of inflammatory condition (cystitis) which produced from decreased frequency of urination, incomplete voiding and urine retention. This condition stimulated the synthesis of different globulin fractions (Stockham and Scott, 2008; Zachary and McGavin, 2017). Hypoalbuminemia which appeared in both the affected groups in comparison to the control group may be due to decrease albumin synthesis under influence of inflammatory cytokines during acute inflammatory condition as the albumin is one of negative acute phase proteins (Stockham and Scott, 2008). Serum albumin value was observed slightly higher in ruptured bladder cases in compare with intact bladder group, possibly due to the higher degree of clinical dehydration due to shifting of the fluid into the peritoneal cavity in the ruptured urinary bladder cases (Mangotra et al., 2017).
Serum AST activity showed highly significant increase in this study in both the affected groups in comparison to the control group could be due to renal tissue injury as the AST activity is high in the cells of kidney tissue. Also, accumulation of excessive amounts of urea and different nitrogenous waste products in the blood may cause uremic syndrome which leads to liver dysfunction ( Jevtovic et al., 2002; Kaneko et al., 2008; Ronco et al., 2009). Serum AST activity was detected in a higher degree in ruptured urinary bladder cases in comparison with intact urinary bladder cases could be due to tearing of the urinary bladder, which a hollow muscular organ and AST enzyme is present in almost of body tissues and its concentration is increased after the occurrence of injury in different types of body muscles (Frandson et al., 2009; Haschek et al., 2013).
Serum ALP activity showed highly significant increase in both the affected groups in comparison to the control group could be due to the renal tubular cells brush border membrane injury and impairment of renal function. Increasing of serum ALP activity is considered as a sensitive marker of renal tubular damage and considers as an early sensitive index for the detection of renal pathological conditions (Leibovitch et al., 1991). Also, increasing of serum ALP activity may be related to cholestatic condition which induced high hepatic ALP isoenzyme activity (Kaneko et al., 2008).
In the present study, serum ALP activity was higher in cases with intact urinary bladder than in ruptured urinary bladder cases possibly due to age–related physiological changes in enzyme activity in the serum of young fast growing animals where predominates isoenzyme from bones. Some variations in the age of animal in both groups may lead to this variation in the enzyme activity (Klinkon and Ježek, 2012).
Hyperbilirubinemia was observed in both the affected groups in comparison to control group could be related mainly to unconjugated hyperbilirubinemia which may resulted from decrease of hepatic uptake of unconjugated bilirubin which known to occur with anorexia and rumen stasis in cattle. Mechanisms of unconjugated hyperbilirubinemia include either increasing of free fatty acids mobilization which interferes with bilirubin uptake by hepatocytes or inadequate availability of glucose for conjugation with bilirubin. Also, some degree of hemolysis may be another possible cause (McSherry et al., 1984; Sharkey and Radin, 2010). This case of hyperbilirubinemia associated mainly with mild elevation in the serum conjugated bilirubin concentration. In cattle, hyperbilirubinemia in most cases is accompanied by an increase of unconjugated bilirubin even with hepatic or post-hepatic obstruction of bile flow (Thrall et al., 2012; Constable et al., 2017). Cases of intact urinary bladder have a higher degree of hyperbilirubinemia than the ruptured urinary bladder cases, which could be due to decreasing degree of pressure on the stretching receptors of urinary bladder wall and lowering back pressure degree after leakage of urine from the ruptured urinary bladder into abdominal cavity and subsequently, reduces pain (Radostits et al., 2007), while in intact urinary bladder group complete blockage of urine flow induce severe pain and subsequent occurrence fully anorexia and rumen stasis so hyperbilirubinemia appears clearly in this group.
In this study, highly significant increase in RBCs count, packed cell volume (PCV) value and hemoglobin concentration (Hb) were observed in both the affected groups in comparison to the control group may be due to dehydration which resulted from decreased water intake by calves and loss of appetite, which the common clinical symptom in all calves. Anorexia leads to dehydration by depriving the water, which produces from food oxidation (Singh, 2005). In addition to previous causes, the increase in theses analytes in bladder ruptured cases may be due to urine accumulation in the abdominal cavity which has low concentration of sodium and chloride, so these electrolytes move from the blood to the abdominal cavity following concentration gradient with water moves out of intracellular, extracellular and intravascular compartments into the abdomen, which creating dehydration and poor circulation (Donecker and Bellamy, 1982; Sockett et al., 1986; Larson, 1996). Lowering of RBCs count and packed cell volume in a ruptured bladder cases in comparison with intact bladder cases may be due to exposure of this group to the high degree of uremic toxins which leads to reducing red blood cells life span and inhibiting of erythropoiesis (Merrill, 2012).
During this study, rupture of the urinary bladder or leakage of urine from highly distended intact urinary bladder leads to infiltration of the peritoneal cavity with urine and subsequently induce peritonitis besides occurrence of different acute inflammatory conditions to the renal system (Din Parrah et al., 2010). Leukopenia which was observed in both the affected groups in comparison to control group may be related mainly to lymphopenia which occurred as a result of the changes in lymphocytes kinetics which stimulated by acute inflammatory mediators. Lymphopenia occurs as a result of increase migration of lymphocytes to inflamed tissue or reducing the rate of lymphocytes moving from lymph node back to the circulation. Mainly, lymphopenia occur in the cases as a result of stress state which associated with an acute inflammatory condition more than the inflammatory process itself (Stockham and Scott, 2008). Neutrophilic, monocytic and eosinophilic leukocytosis were observed in both the affected groups in compare to control group may be due to acute inflammatory condition which led to induces inflammatory mediators to enter the circulation and stimulate bone marrow to produce neutrophils, cytokine stimulation of monocytes production and release to circulation and highly release of eosinophils to perform anti-inflammatory functions or increase its attraction to injured tissue after mast cell degranulation (Robertson and Seguin, 2006; Stockham and Scott, 2008).
On the inflammatory leukogram, countless leukogram changes occur, which reflect the duration and severity of the disease condition and range of body tissue involvement. Neutrophils count may vary from low to high during inflammatory conditions, depending on the balance between the rate of emigration of blood neutrophils to different tissues in response to increasing chemoattractants, and the bone marrow granulopoiesis rate to replace the blood neutrophils, so the low neutrophils count in the cases with a ruptured bladder in comparison with intact bladder cases may be due to fast movement of neutrophils into an wide area of inflammation and high tissue needs for neutrophils which lead to depletion of storage pool in bone marrow. The monocytes and neutrophils share a common stem cell (colony-forming unit [CFU]-GM), so the change in monocytes count may parallels the change in neutrophils count either with increase or decrease the count (Gaunt, 2004). Also, monocytes don’t have any significant storage pool in the bone marrow so the high tissue demands in inflammatory condition may decline its count (Handin, 2003). Wide area of injured tissues in ruptured bladder group may induce a high rate of mast cell degranulation and attract more eosinophils so its count higher in this group in compare with intact bladder group.
CONCLUSIONS
In perspective of findings of this study, buffalo calves with ruptured urinary bladder recorded severe alterations in most of biochemical and hematological analytes in comparison to the intact bladder group. So these cases need a quick surgical and therapeutic intervention before die from severe uraemia and electrolytes disturbance (poor prognosis). Clinical examination and ultrasonography consider as a first diagnostic tool but don’t clarify the possible complications. The laboratory tests are more reliable indicator for measurement the degree of disturbance and possible prognosis besides differentiating cases of ruptured bladder from intact bladder cases and other diseases that result in accumulation of intra-abdominal fluids.
ACKNOWLEDGMENTS
The author expresses their sincere thanks to the valuable assistance rendered by Ahmed Monir Attia, assistant lecturer in Department of Surgery, Anesthesia and Radiology, Faculty of Veterinary Medicine, Zagazig University, Egypt to complete the work.
CONFLICT OF INTEREST
The author declare that there is no conflict of interest.
Authors Contribution
Hager TH Ismail designed and performed the study, analyzed the data, discussed the results, wrote and revised the manuscript. The manuscript was critically revised for important intellectual content and approved as the final manuscript by me.
REFERENCES





