Advances in Animal and Veterinary Sciences
Research Article
Seroprevalence of Anaplasmosis in Dairy Cattle from Peninsular Malaysia
Asinamai Athliamai Bitrus2,5, Yusuf Abba2,4, Faez Firdaus Abdullah Jesse1,3*, Joy Lee Xing Pei1, Innocent Damudu Peter1,4, Idris Umar Hambali1,4, Abd Wahid Haron1, Mohd Azmi Mohd Lila1, Abdul Aziz Saharee1
1Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 2Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 3Institute of Tropical Agriculture and Food security, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 4Faculty of Veterinary Medicine, University of Maiduguri. P.M.B 1069 Maiduguri, Borno Nigeria; 5Department of Veterinary Public heath, Faculty of Veterinary Science, Chulalongkorn University, 10330 Pathumwan, Bangkok.
Abstract | Bovine anaplasmosis also known as Red water or Gall sickness is an important disease of cattle primarily caused by Anaplasma marginale and it infects erythrocytes, which results to erythrophagocytosis and subsequently anaemia. This study was carried out to determine the seroprevalence of bovine anaplasmosis among dairy cattle in some randomly selected ruminant farms in Peninsular Malaysia. A total of 45 Blood samples were collected via jugular venipuncture from cattle from four (4) farms using convenient sampling technique. Twelve(12) cows were sampled from each of farms A and B, while 13 cows were sampled from farm C and 8 from Farm D. Heparinized whole blood was used to prepare Giemsa-stained thin blood smears for microscopic detection of anaplasmosis. Serum was extracted from coagulated blood for serological testing using Anaplasma antibody Test Kit (VMRD, Inc. United State of America). The result showed an overall seroprevalence rate of 51.11% (23/45). Farm level seropositivity showed 83.3% (10/12), 41.7% (5/12), 23.1% (3/13) and 62.5% (5/8) for Farms A, B, C, and D, respectively. Age specific seroprevalence showed a 53.13% (17/32) in cows that are more than 3 years old and 46.15% (6/13) in cows aged between 1-3 years. Additionally, a significant (p<0.05) association between microscopic detection and serological detection method was observed. . In conclusion, a high seroprevalence rate was observed in the selected ruminant farms.
Keywords | Bovine anaplasmosis, Anaplasmamarginale, Seroprevalence, cELISA, Microscopic detection.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 30, 2017; Accepted | January 23, 2018; Published | January 29, 2018
*Correspondence | Faez Firdaus Abdullah Jesse, Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; Email: jesseariasamy@gmail.com
Citation | Bitrus AA, Jesse FFA, Abba Y, Pei JLX, Peter ID, Hambali IU, Haron AW, Lila MAM, Saharee AA (2018). Seroprevalence of anaplasmosis in dairy cattle from peninsular Malaysia. Adv. Anim. Vet. Sci. 6(2): 70-74.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.2.70.74
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Jesse et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Agriculture is considered to be one of the bedrock of Malaysian economy, contributing about 8.9% to the country’s gross domestic product (GDP) (Jabatan Perangkaan Malaysia, 2016). Dairy farming, an important component of agriculture in Malaysia, is a major contributor to the country’s economy. Over the years, there is a gradual increase in local fresh milk production in Malaysia. There was an increase in local milk production from 45.5 million litres in 2006 to 76.0 million litres in 2015, this represents an estimated 67.03% increase in local milk production. For instance, in 2006, the country recorded a 67.03% increase in local milk production level from 45.5 million litres annually to 76.0 million litres in 2015. However, this is still not sufficient to meet local demand as milk is imported to meet this shortfall (Agrofood Statistic, 2012).To ensure improvement in the dairy industry, control of diseases in cattle is of paramount importance. In Malaysia, anaplasmosis is one of the most common disease affecting local cattle production.
Bovine anaplasmosis is a severe disease of cattle, characterised by haemolytic anaemia and causes significant economic loss (Ashuma et al., 2013). The losses are as a result of reduction in milk production, increased cost of treatment and management of the disease condition. The disease, although normally causes sporadic mortalities may result in high morbidity when herd immunity is compromised (Abba et al., 2016). Anaplasmosis in cattle is caused mainly by Anaplasma marginale and are intra-erythrocytic microorganisms of the order of Rickettsiales. Two main species of concern are Anaplasma marginale and Anaplasma centrale, with the former being more pathogenic (Abba et al., 2016). Clinical signs of anaplamosis include fever, jaundice, anorexia and lethargy, which can lead to a dramatic decrease in milk production (Smith, 2015). Severity of the disease is related to various factors such as virulence of the strain, age-related host susceptibility and breed resistance. Animals that recover from the disease may remain carriers for life and thus becoming reservoirs for transmission to other susceptible hosts. For effective control of anaplasmosis, early diagnosis and treatment is essential, while continuous screening should be practiced to control the disease (Smith, 2015).
In earlier studies, Pong et al. (2012) reported 87% and 60% prevalence of anaplasmosis in Peninsular and East Malaysian cattle, respectively; revealing its endemicity in Malaysian cattle. The current seroprevalence rate of Anaplasma in most small holder cattle farms in Peninsular Malaysia is unknown and this could be a serious threat to cattle health and production in this area. Thus, this study was undertaken to determine the seroprevalence of bovine anaplasmosis among dairy cattle in these farms.
MATERIAL AND METHODS
Blood Sampling
A total of 45 cows between the ages of 2 to 9 years old were randomly selected from four farms in Selangor, Malaysia using convenience sampling technique. Ten milliliters (10mL) of blood was obtained from each cow via jugular venipuncture into pre-labelled vacutainer tubes (5mL each into plain and sodium heparin tubes to harvest serum and plasma, respectively) and transported in an ice box to the laboratory for serology and microscopy. The study was performed according to the guidelines for the care and use of animals as approved by Institutional Animal Care and Use Committee of Universiti Putra Malaysia, Animal Welfare Act (2014) [AUP No.: FYP.2016/FPV (32.50)].
Serum Extraction
Blood samples collected for serology were centrifuged at 3000 × g for 5 minutes to harvest serum which was then collected and transferred into a well labelled 1.5 mL micro-centrifuge tubes for storage at -20 oC.
Serological Testing
The serum samples were analysed using the Anaplasma Antibody Test Kit cELISA v2 (Veterinary Medical Research and Development) according to manufacturer’s instruction. Briefly, conjugate solution was prepared by diluting 1:99 of 100X Antibody-peroxidase Conjugate concentrate and Conjugate Diluting Buffer. Wash solution was prepared by diluting 1:9 of 10X Wash Solution Concentrate and distilled water. The positive control was loaded in duplicates, while negative control were loaded in triplicates prior to loading of serum samples followed by 50 µL of thawed serum sample loaded in duplicates into the remaining wells. The plate was then incubated at room temperature for one hour, followed by double washing with diluted wash solution. Fifty 50 µL of diluted Antibody-Peroxidase conjugate was then loaded into each well and then incubated at room temperature for 20 minutes, and finally washed for four times with diluted wash solution. Fifty 50 µL substrate solution was then added into each well. The plate was re-incubated at room temperature for 20 minutes before immediately stopping the reaction with 50 µL of Stop Solution. The plate was immediately read using a microplate absorbance spectrophotometer (Infinite® M200 from Tecan Trading AG) at a wavelength of 630nm.
Test Validation and Results Interpretation
The tests were validated by using negative and positive controls. Tests are valid when the mean optical density (OD) of the negative controls was > 0.40 and
≤ 2.10 while the mean of positive controls ≥ 30% inhibition.
Percentage inhibition (I%) was calculated using the following formula:
I% = 100 [1- (ODSample / ODNegative control]
Where ODSampleis the OD of the sample and ODNegative control the OD of the negative control.
Test samples with < 30% of inhibition are negative for bovine anaplasmosis, while those with ≥ 30% inhibition are positive.
Thin Blood Smear Preparation and Microscopic Examination
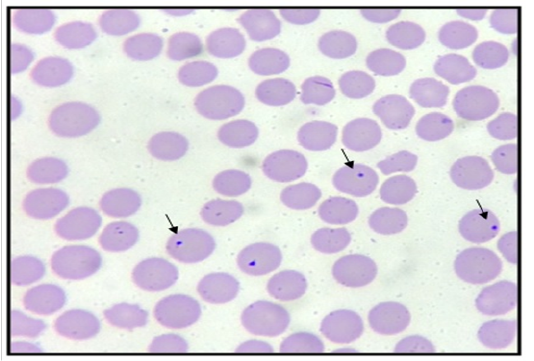
Blood samples from heparinised tubes were used to prepare thin blood smears. Thin blood films were air-dried, fixed with absolute methanol for 30 seconds and stained with Giemsa-stain using standard procedure as described by OIE (2015). The slides were examined under oil-immersion light microscope. The Anaplasma marginale was identified as dense, rounded and deeply stained intra-erythrocytic bodies with a diameter of approximately 0.3 – 1.0 µm, while Anaplasma centrale have a similar appearance at a more central location in the erythrocyte. A total of at least 50 fields at the feather edge of the blood smear of each slide were examined. A blood smear was considered to be positive when the organism was visualized and identified.
Packed Cell Volume (PCV) Determination
The heparinized blood samples were thoroughly mixed on a tube rotator. The hematocrit capillary tube was filled to at least three-quarter full, before sealing one end of the capillary tube. The capillary tubes were spun in amicrohaematocrit centrifuge (Haematokrit 20, Hettich Instruments) for 5 minutes, and the PCV determined by the standard method. In this study, the PCV was assigned “Low” and “Normal” when the value is < 0.24 and ≥ 0.24 L/L (Bull et al., 2003) respectively.
Statistical Analysis
Chi-square test was used to analyze the data using IBM SPSS Statistics Version 22.0 to determine association between age and PCV level with seropositivity, as well as to compare between the serological and microscopic detection methods. A p value of p<0.05 was considered as significant.
RESULTS
Seroprevalence of Bovine Anaplasmosis
The result of this study showed that a total of 23/45 (51.11%) of all the dairy cows screened were seropositive for bovine anaplasmosis (Table 1). Seropositivity to bovine anaplasmosis is seen more in dairy cows between the ages of 3 years and above than in cows less than 3years of age (Table 2). However, there was no statistically significant association (p>0.05) between the age of cow and seropositivity. In addition, (8.89%) 4/45 of the cows had low PCV levels, whereas, only 25%(1/4) of the cows with low PCV level were seropositive (Table 3). A high percentage (53.66%) of cattle were seropositive despite having normal PCV levels. However, there was no statistically significant association between the PCV level of cows and its seropositivity at p > 0.05. Microscopic detection showed that 16 samples were positive for Anaplasma marginale. However, only (75%) 12/16 of the samples were seropositive for bovine anaplasmosis. Among the seropositive animals, only 52% was detected as positive by the microscopic detection method (Table 4) (Figure 1).
The Anaplasma antibody cELISA test kit used has a sensitivity of 95% and specificity of 98%. The sensitivity and specificity of thin blood smear detection method in this study was calculated based on the formula of Thrusfield (2007):
Sensitivity = (True Positive/Total diseased) x 100
Specificity = (True negative/Total non-diseased) x 100
Based on this formula, the sensitivity and specificity of the microscopic detection method was 52.17% and 81.82%, respectively, showing that the serological is more sensitive and specific than the microscopic detection method.
Table 1: Seroprevalence of bovine anaplasmosis among dairy cows in some farms in Peninsular Malaysia.
| Farm | Number tested | Number positive | Percentage (%) |
| Farm A | 12 | 10 | 83.33 |
| Farm B | 12 | 5 | 41.67 |
| Farm C | 13 | 3 | 23.98 |
| Farm D | 8 | 5 | 62.50 |
| Total | 45 | 23 | 51.11 |
True prevalence is estimated from apparent prevalence using the following formula (Thrusfield, 2007):
True prevalence = 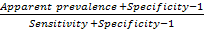
In the farms sampled, the true prevalence of bovine anaplasmosis was found to be 52.81%.
Table 2: Seroprevalence of bovine anaplasmosis based on age among dairy cattle in some cows farms in Peninsular Malaysia.
| Age (years) | Number tested | Number tested | Percentage (%) |
| < 1 | 0 | 0 | 0.00 |
| 1 – 3 | 13 | 6 | 46.15 |
| > 3 | 32 | 17 | 53.13 |
| Total | 45 | 23 | 51.11 |
Table 3: Seroprevalence of bovine anaplasmosis according to PCV result among cows in a some selected farms in Peninsular Malaysia.
| Haematocrit result (PCV) | Number tested | Number positive | Percentage (%) |
| Low (<0.24 L/L) | 4 | 1 | 25.00 |
| Normal (>0.24 L/L) | 41 | 22 | 53.66 |
| Total | 45 | 23 | 51.11 |
Table 4: Comparison between serological method (cELISA) and microscopic detection methods
| cELISA | |||
| Microscopic detection | Positive (n) | Negative (n) | Total (n) |
| Positive | 12 | 4 | 16 |
| Negative | 11 | 18 | 29 |
| Total | 23 | 22 | 45 |
| n = number | |||
Discussion
Bovine anaplasmosis caused by A. marginale is the major cause of morbidity and mortality in the tropics and subtropics, particularly in exotic and crossbred cattle. The geographic distribution of the disease is however dependent on the density and distribution of tick vectors and reservoir host (Singh et al., 2012). The most commonly used laboratory method for the identification of the organism in most developing countries, is microscopic examination of Giemsa stained thin blood film. However, this method cannot detect low level of rickettsiaemia as seen in infected host. In addition, in persistently infected cattle, it is difficult to differentiate the pathogen from similar structures such as Howell-Jolly bodies, Heinz bodies and staining artifacts, thus rending this method unreliable (Noaman and Shayan, 2010; Singh et al., 2012). Other alternative techniques for diagnosis of anaplasmosis include serological test (ELISA) and/or PCR technique. However, in this study, we employed the use of both ELISA and microscopic examination of thin blood films to compare the two methods in determining the prevalence of anaplasmosis in some selected cattle farms in Peninsular Malaysia. The result obtained showed an overall prevalence rate of 51.1% in all samples tested (Table 1). This result is higher than the initial reported prevalence 45.2% of bovine anaplasmosis by Singh et al. (2012) where the authors amplified a 458 bp specific for msp5 for A. marginale. However, a detection rate of 45.2% to 73.1% was recorded when nested PCR was performed. Thus, indicating that nested PCR increased the detection rate and serves as a better alternative for the detection of BA than other conventional detection methods. Additionally, the difference in the seroprevalence rate can be attributed to diagnostic method used, breed and age of cattle sampled as well as the type of management systems operated by the farms. In Brazil, Da Silva et al. (2014) reported a 49% seroprevalence of bovine anaplasmosis in water buffaloes from 16 provinces while only 5.4% (27/500) were positive for PCR detection of A. marginale in the blood. The authors further reported that the sex and geographical location of the animal significantly influenced the outcome of the disease. In this study, only dairy cows were sampled and used. However, age-related seropositivity was observed. High seropositivity to bovine anaplasmosis was observed in dairy cows that are greater than 3years old than in cows between the ages of 1 to 3 years old and the result is not statistically significant (p>0.05, Table 2). This finding was however not in agreement with the work of Alfredo et al. (2005), where the authors reported a high seroprevalence 63% of bovine anaplasmosis among calves in Tete province of Mozambique. However, the authors also corroborated the findings of Da Silva et al. (2014) indicating that higher seroprevalence of bovine anaplasmosisis associated with geographical location as well as the sex of animals. It is important to note that, despite the difference in the number of samples used, the serological test applied in both studies also differs. In addition, using the modified card agglutination test, Hungerford and Smith (1997) also reported that in both low and high risk areas, the seroprevalence of bovine anaplsmosis increased with increase in the age of the cattle. This finding is in agreement with the report of this study where, high seropositivity was observed in dairy cows that are more than 3 years of age, while a lower rate was observed in those between the ages of 1-3 years of age. In South Africa, Mutshembele et al. (2014) reported 65% to 100% prevalence of A. marginale. The authors also reported that there is a correlation between the genetic diversity and prevalence of A. marginale. Furthermore, In Madagascar, Pothmann et al. (2016) reported a high prevalence of bovine anaplasmosisi n ticks and cattle. The authors also detected a high genetic heterogeneity among strains and low clinical manifestation of bovine anaplsmosis thus, confirming the stability of the organism in endemic areas. Similarly, Hamou et al. (2012) reported a seroprevalence of 16.5% after sampling n=668 blood samples from cows. The authors also found a relationship between geographic location and month of sampling.
In this study, (4/45) of the cattle were reported to have low PCV levels and only 25% (1/4) of the cattle with low PCV level was seropositive to bovine Anaplasma (Table 3). Interestingly, a high percentage (53.66%) of dairy cows that are seropositive have normal PCV levels. However, there was no statistically significant association between the PCV level of cattle and its seropositivity at p . >0.05. Thus indicating that low PCV levels does not correlate to seropositivity. Additionally, this might be because of the presence of carriers among the dairy cows, thus serving as reservoirs of infection. Similar finding was also reported by Hornok et al. (2012) where the authors reported the detection of 92% carrier state in an outbreak of anaplasmosis in Hungary. It was also observed that although 16 samples were positive for Anaplasma using microscopic detection in this study only (12/16) of the samples were seropositive for bovine anaplasmosis. Among the seropositive animals, only 52% were detected as positive by the microscopic detection method (Figure 1) (Table 4).
Conclusion
This study has shown a high seroprevalence of antibodies to anaplasma spp in cattle. Higher seroprevalence was found in cows with 3 years and above. It is recommended that studies with a larger sample size and a bigger study area will be required to elucidate on the true seroprevalence rate of bovine anaplasmosis in peninsular Malaysia. There is also need to identify the genotype (s) of Anaplasma circulating in cattle population so as to use appropriate vaccine for prevention of anaplasmosis in cattle.
Acknowledgements
The authors wish to appreciate the management and staff of the University Veterinary Hospital (UVH) of Universiti Putra Malaysia (UPM) for their technical assistance during the course of this study.
Conflict of Interest
The authors have none to declare.
AuThors Contribution
All authors contributed equally.
References