Advances in Animal and Veterinary Sciences
Case Report
Suppurative Pneumonia and Lymphadenitis in a Goat Associated with Infection by Corynebacterium Pseudotuberculosis– A Case Study
Rahul Singh1*, Swati Kumari1, Jay Prakash Yadav2, Shivvaran Singh3, Ashok Kumar4, Susil Kumar5
1Division of Pathology, ICAR- Indian Veterinary Research Institute, Izatnagar, Bareilly-243 122 (U.P), India; 2Division of Veterinary Public Health, ICAR-Indian Veterinary Research Institute, Izatnagar (U.P.)-243122, India; 3Division of Bacteriology and Mycology, ICAR-Indian Veterinary Research Institute, Izatnagar (U.P.)-243122, India; 4Division of Virology, ICAR-Indian Veterinary Research Institute, Izatnagar (U.P.)-243122, India; 5Division of Animal Genetic and Breeding, ICAR-Indian Veterinary Research Institute, Izatnagar (U.P.)-243122, India.
Abstract | A case of suppurative pneumonia with inflammation of lung-associated lymph node (mediastinal and bronchial) caused by Corynebacterium pseudotuberculosis was described in a 2-year-old Shirohi breed, the female goat which presented clinical sign, respiratory distress, nasal discharge with fever followed by sudden death. The suppurative inflammation was detected in lung parenchyma and lymph node was characterized by formation of caseo-calcified nodules of multiple abscesses which were scattered throughout lung lobes and even in the chest wall. The prominent enlargement of mediastinal and bronchial lymphnodes were sectionedand analysis revealed laminated calcified granules and semi-solid fluid with greenish yellow inspissated pus giving the spherical onion-skin appearance (pathognomonic signs) respectively. Histopathologically,the lung section was characterized by a central caseo-necrotic core admixed bacterial colonies and infiltration of polymorpho nuclear cells, few mononuclear cells, lymphocytes, plasma cell and macrophages. The bacterial culture examination showed the presence of bacterial growth forming cream white, dry, waxy colonies with a narrow zone of β-haemolysis. They were stained gram positive cocco-bacilli arranged in Chinese pattern. The extracted DNA from tissue was further confirmed by polymerase chain reaction (PCR) assay for amplified pld gene specific for goat. Present casestudy detail described the pathognomonic gross, histopathological lesions ofC. pseudotuberculosis in a goat with bacteria isolation and finally confirmed by PCR.
Keywords | Suppurative pneumonia, PCR, Histopathology, Lymphadenitis, Goat
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 24, 2017; Accepted | August 23, 2017; Published | September 29, 2017
*Correspondence | Rahul Singh, Division of Pathology, ICAR- Indian Veterinary Research Institute, Izatnagar-243 122 (U.P) India; Email: drrahul.sigh1987@gmail.com
Citation | Singh R, Kumari S, Yadav JP, Singh S, Kumar A, Kumar S (2017). Suppurative pneumonia and lymphadenitis in a goat associated with infection by corynebacterium pseudotuberculosis– A case study. Adv. Anim. Vet. Sci. 5(10): 405-409.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.10.405.409
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Rahul Singh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Caseous lymphadenitis (CLA) or pseudotuberculosis is chronic disease of sheep and goats of worldwide distribution with high prevalence in flock that is caused by Corynebacterium pseudotuberculosis (previously know Corynebacterium ovis) (Batey, 1986; Stackebrandt et al.,1997; De Sá Guimarães et al., 2011; Ali et al., 2016). This bacteria is also isolated from ahorse, llama, alpaca, buffalo, deer, camelsand laboratory animals (Dorella et al., 2006; Braga 2007; Baird and Fontaine, 2007; Chakraborty et al., 2014) rarely humans thus, it is considered an occupational zoonosis (Join-Lambert et al., 2006). The genus Corynebacterium is a gram positive, small or pleomorphic coccobacillus, spore-forming and the microaerophilic organism that belongs to family Corynebacteriaceae, class Actinobacteria (Collet et al., 1994). CLA is characterized by multiple abscesses formation that may be internal or superficial organsand different lymph node like mandibular, superficial cervical, sub-iliac, popliteal, or mammary lymph nodes (Dorella et al., 2006). However, in some cases, the disease may be subclinical without showing any external symptom formation of internal multiple abscesses in lungs and associated lymph nodes (bronchial and mediastinal lymph node), which may cause severe chronic suppurative pneumonia (Binns et al., 2007; Baird and Fontaine, 2007; De Sá Guimarães et al., 2011). The disease causes significant economic losses, from the condemnation of skins and carcasses because of abscesses, to significant losses in reproductive efficiency, wool, meat and milk production in small ruminants. The transmission of disease to others animals in flocks mainly from nasal, oral discharge or lacerated superficial lymph node abscess are very important source of infection of C. pseudotuberculosis (Radostits et al., 2007; Fontaine and Baird, 2008; Sunil et al., 2008).
Phospholipase D (PLD) is an exotoxin of this bacterium which acts as virulence factor that increases vascular permeability and bacterial survival in the host. It is essential for the dissemination of the bacteria from the location of the primary infection (local lymph node) to other organs (lungs, regional lymph nodes, mesenteric lymph nodes, etc.), because it causes the lysis of cell membranes, rich in phospholipids (sphingomyelin), causing microhemorrhages and vascular lesions, with increased vascular permeability (Dorella et al., 2006; De Sá Guimarães et al., 2011).
Diagnosis is based on characteristics of the pathological lesions (presence of external abscesses), and this confirmation diagnosis,isolation, and biochemical identification, mainly to differentiate other pathogens that also cause abscesses, such as Arcanobacterium pyrogens and Pasteurella multocida (Dercksen et al., 2000; Dorella et al., 2006). PCR diagnosis using specific primers for C. pseudotuberculosis, which is a significant role in the control of disease outbreaks in any regions (Çetinkaya et al., 2002). The disease occurs worldwide, and severe issues due to it have been described in commercial flocks of many different countries (Arsenault et al., 2003; Fontaine and Baird, 2008).
The aim of present study includes the diagnosis of suppurative pneumonia with lymphadenitis in naturally affected goat caused by C. pseudotuberculosis. We identified the pathogonomic gross and histopathological lesions in the lung and associated lymph node with microbiological isolation and PCR detection in tissue samples collected during necropsy.
MATERIAL AND METHODS
Case History
A 2-year-old female goat, Shirohi Indian breed was presented for post-mortem at Division of Pathology, ICAR- Indian Veterinary Research Institute, Bareilly in India from the farm. History was presented by farm incharge of ICAR- Indian Veterinary Research Institute. The presented clinical sign, respiratory distress, nasal discharge with fever followed by sudden death.
Pathological Examination
On necropsy, the carcass body conditions was not good. The carefully examined of the carcass for any gross pathological lesions in various organs. The appropriate representative samples (0.5 cm thick) tissue samples from affected organs were collected in 10% buffered neutral formalin (NBF) fixative for histopathology. The paraffin blocks of the tissues were then cut into 4 -5 μm thick paraffin sections by microtome and stained with hematoxylin and eosin technique (H&E).
Bacteriological Examination
For bacterial isolation, a loopful of the pus was inoculated into nutrient agar, 5% sheep blood agar and MacConkey agar and incubated aerobically at 37°C for 24-48 hours. The isolates were identified according to the colony morphology, Gram’s stain, as well as biochemical characters. For identification of C. pseudotuberculosis, the isolates were further inoculated on cystine tellurite blood agar and incubated at 37°C for 48 hours. The viable tissue samples lung and lymph node were also collected aseptically in sterile, screw capped polypropylene vials using sterile scissors and forceps stored at -800C for PCR diagnosis. Immersion smears were also taken in the clean slide for Gram s’ staining.
Pcr and Dna Extraction
Genomic DNA from lung samples was extracted using DNA extraction kit (Qiagen) as per manufacturer’s protocol and PCR carried out by using forward primer ATAAGGTAAGCAGGGAGGA and reverse primer ATCAGCGGTGATTGTCTTCC specific to pld gene for C. pseudotuberculosis. PCR was carried out as described previously (Pacheco et al., 2007) and the amplified products at each round of PCR were detected by electrophoresis and visualized by UV gel documentation system.
Results and Discussion
Grossly, the suppurative lungs were red to gray, solid and pale with suppurative exudates in the bronchi and bronchioles. Small pea to the walnut size of multiple abscesses (about 10-12 discrete nodules) were scattered throughout the lung parenchyma. Caseo-calcified nodules were found of variable sizes in different lobes and even in the chest wall (Figure 1A and Figure 1B). The prominent enlargement of mediastinal and bronchial lymph nodes with completely converted into abscesseswith calcified granules recorded in bronchial lymph node (Figure 2A).The cut surfaces of themed iastinallymph nodewas showing thick semi- solid fluid with greenish yellow inspissated pus in lymph node and across section giving the spherical onion-skin appearance (pathognomonic signs of CLA) of abscess (Figure 2B).

Figure 1 Corynebacterium pseudotuberculosis in goat: A) Caseo-calcified nodules of variable sizes in different lobes of pneumonic lungs and even in the chest wall (arrows); B)Small pea to the walnut size of abscesses with greenish pus scattered throughout the lung parenchyma (arrows)
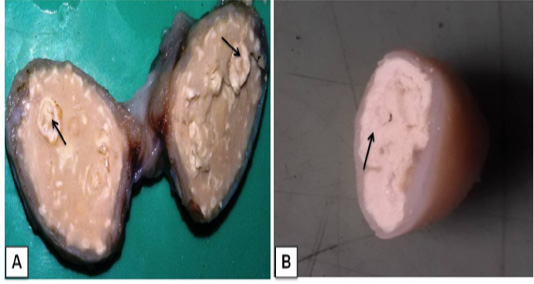
Figure 2 Lymphadenitis in goat. A) A cross-sectional view of affected bronchial lymph node hard calcium granules that deposited with abscesses (arrows); B) The mediastinal lymphnode showedcharacteristic lamellate (onion skin or onion ring), pathognomonic signs of CLA (arrow).
In the present study, gross lesions finding in lung and associated lymph node are similar lesions described variousworker previously in goat (Radostits et al., 2007; Sonawane et al., 2016). In our study, media stinal lymph node cut section given pathogonomic lesion for pseudotuberculosis in goat. This finding agreement to previously reports (Fontaine and Baird, 2008; Radostits et al., 2007). Mahmood et al. (2015) described lung lesion in experimentally induced infection by C. pseudotuberculosis in goat, which showed similar finding to present study.
Histopathologically, focal areas of suppuration with multiple necrotic areas were observed in lung parenchyma. The suppurative areas were characterized by a central case-onecrotic core surrounded by apyogenic membrane with infiltration of polymorpho 3.n+-uclear cells few mononuclear cells, lymphocytes, plasma cell and macrophages. Vascular congestion and inflammatory exudates were seen in the alveoli of the affected part lung (Figure 3A). The purulent material was present in bronchi and bronchioles along with the infiltration of neutrophils. Most of the cases had caseo-purulent material admixed with bacterial colonies and surrounded by thick connective tissue capsule and fibro-vascular reaction. (Figure 3B).
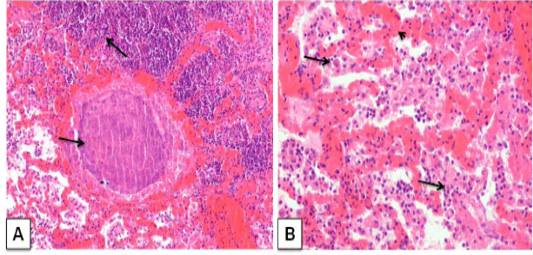
Figure 3: Corynebacterium pseudotuberculosis abscess in lung section of a goat. ; A) caseative necrotic mass with bacterial colonies within the lung parenchyma (arrows); H&E. (X 100) B) Lung section showing vascular congestion (arrow head), infiltration of neutrophils withmacrophage in the alveoli (arrows). H&E. (X 200)
The results of this study were consistent with those reported by (Radostits et al., 2007; Sonawne et al., 2016; Singh et al., 2017) who stated the classical presentation of the histopathological feature of the pyogranulomatous inflammation in lungs. Osman et al. (2012) described similar histopathological lunglesion in a mouse model, lungs showed congestion, hemorrhage, and development of micro-abscesses, caseous necrosis and presence of tubercular like granuloma that surrounded with neutrophil and macrophage.
The bacterial culture examination showed the presence of bacterial growth forming cream white, dry, waxy colonies with a narrow zone of β-haemolysis. They were stained gram positive cocco-bacilli arranged in Chinese pattern indistinguishable from C. pseudotuberculosis (Figure 4A). Impression smear of heart blood, lung, and lymph node were showed gram positive cocco-bacilli arranged in Chinese pattern (Figure 4B) These results are in agreement with previous studies (Fontaine and Baird, 2008; Radostits et al., 2007). Chirino-Zárraga et al. (2006) have described the bacteriological characterization of C. pseudotuberculosis from18 Venezuelan goat flocks,which agreement the present study of bacterial isolation in goat.
The extracted DNA from tissues (lung and associated lymph node) was subjected to PCR amplification with specific primers that annealed at the pld gene of the C.pseudotuberculosis genome, and amplified an area of 203bp and confirmed. There was no amplification in the controls (Figure 5). The present of molecular diagnosis by using PCR for pld gene is agreement with previously studies (Cetinkaya, et al., 2002; Pacheco et al., 2006; Ali et al., 2016).
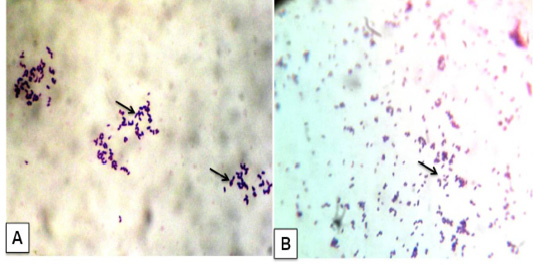
Figure 4: Corynebacterium pseudotuberculosis in goat: A) Bacterial culture gram’ staining showed gram positive cocco-bacilli arranged in Chinese pattern; B) Impression smear from heart also given gram positive cocco-bacilli arranged in Chinese pattern. (x1000)
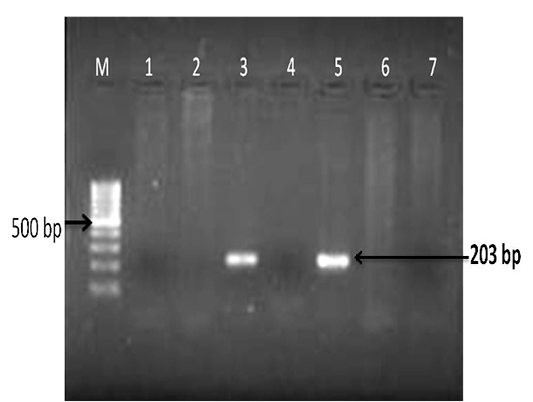
Figure 5: Detection of Corynebacterium pseudotuberculosis DNA in affected lung and lymph node tissue samples: PCR for pld gene regions; Lanes- 3 and 5 are positive amplification (203 bp); Lanes- 1, 2, 4 and 6 are negative; Lane 7- negative control and M-100 bp ladder.
Conclusions
The present study concludes that the pathomorphological, bacteriological and PCR techniques could be help in the diagnosis of C.pseudotuberculosis in small ruminants. The extensive damage to the lung caused by multiple foci of the abscesses and associated lymph node may be the systemic spread of the infection in internal organs, couldbe the reason for emaciation and death of the goat understudy.
Acknowledgments
The authors are thankful to the Director ICAR-IVRI for providing necessary facilities for research work. The first author is grateful to the ICAR, New Delhi for providing the Junior Research Fellowship.
Conflict of interest
We declare that there is no conflict of interest.
Authors Contribution
All the authors have contributed equally in terms of giving their technical and scientific knowledge to written the article.
REFERENCES





