Advances in Animal and Veterinary Sciences
Research Article
Change in Viability, Motility and Membrane Integrity of Spermatozoa After Equilibration and Thawing in BHT Added Bovine Semen
Anoop Singh, Mridula Sharma*, Shiv Prasad
Department of Veterinary Gynaecology and Obstetrics,College of Veterinary and Animal Sciences, G.B Pant University of Agriculture and Technology, Pantnagar-263145, Uttarakhand, India.
Abstract | This study was conducted to evaluate effect of butylated hydroxytoluene (BHT) on reduction in semen quality (progressive motility, viability and membrane stability) from dilution to equilibration and thawing. Thirty-six ejaculates from two bulls were individually collected and extended with glycerolated egg yolk Tris diluter. Samples were divided into three groups according to BHT concentration as 0 mM (C) 0.5 mM (T1) and 1.0 mM (T2). Reduction in the progressive motility, sperm viability and membrane integrity was observed from Stage I (after dilution) to Stage II (post equilibration) and Stage III (post thaw). Decrease in percentage individual motility from Stage I to Stage II, Stage II to Stage III and Stage I to III was lowest in group T2 though it was non-significant. Decrease in per cent viability from Stage I to Stage II, Stage II to Stage III and Stage I to Stage III indicated lesser loss in viability in group T2. Percent decrease in membrane stable spermatozoa from Stage I to Stage II, Stage II to Stage III and Stage I to Stage III was minimum in group T2. Addition of 1mM of BHT in semen dilutor showed better results as fall in sperm viability, progressive motility and membrane integrity was least from post dilution to post equilibration and post thaw though it was non-significant. Thus experiments to observe the effect of BHT on post thaw semen quality should be conducted at large scale.
Keywords | Sperm, Butylated hydroxytoluene, Membrane integrity, Antioxidant, Crossbred bovine spermatozoa.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 26, 2017; Accepted | July 27, 2017; Published | August 10, 2017
*Correspondence | Mridula Sharma, Department of Veterinary Gynaecology and Obstetrics,College of Veterinary and Animal Sciences, G.B Pant University of Agriculture and Technology, Pantnagar-263145, Uttarakhand, India; Email: sharmavetmridula@yahoo.co.in
Citation | Singh A, Sharma M, Prasad S (2017). Change in viability, motility and membrane integrity of spermatozoa after equilibration and thawing in bht added bovine semen. Adv. Anim. Vet. Sci. 5(8): 329-333.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.8.329.333
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Singh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Cryopreservation is detrimental to sperm function and fertility even with the most-up-to date techniques. Sperm viability and fertility is decreased by about 50% after cryopreservation (Lessard et al., 2000). Physiological levels of reactive oxygen species (ROS) play essential roles in sperm capacitation, acrosomal reaction and fertilization process (Bailey et al., 2000). However, higher production of ROS due to oxidative stress during freezing and thawing of bull semen is responsible for the deterioration of semen quality (Bilodeau et al., 2000). The damage due to increased ROS can occur in sperm bio-membrane system due to presence of high content of polyunsaturated fatty acids (Kankofer et al., 2005). Increased ROS affect motility, plasma membrane integrity, viability and acrosomal integrity (Bilodeau et al., 2001; Lenzi et al., 2002) which ultimately reduce the fertilization potential of the spermatozoa. The sperm plasma membranes are highly susceptible to lipid per oxidation by oxygen free radicals and H2O2 (Baumber et al., 2003; Ogretmena and Inanan 2014). Free oxygen radicals in the sperm cells play a major role in lipid per oxidation as well as in protein damages that lead to cell death (Shoae and Zamiri 2008) or altered characteristics (DNA and sperm membrane damages). Concentration of naturally present antioxidant is usually decreased during the cryopreservation process by dilution of semen with extender and excessive generation of ROS molecules (Roca et al., 2004) and its harmful effect to sperms is not nullified by naturally present antioxidant. Supplementation of natural or synthetic antioxidants in semen extender would be beneficial for the improvement of post-thawed semen characteristics (Jamadia et al., 2016).
Butylated hydroxytoluene (BHT) is also an excellent antioxidant and behaves as a synthetic analogue of vitamin E, primarily acting as terminating agent that suppresses autoxidation by converting peroxy radicals to hydroperoxides (Fujisawa et al., 2004). Irrespective of vitamin E the BHT, being lipid soluble, acts as an antioxidant within and outside the sperm membrane. Therefore, it easily penetrates the sperm membrane, decreases the ice-crystal formation within the cell and protects the sperm (Naijian et al., 2013).
Thus, the present study was planned to evaluate the effect of butylated hydroxytoluene (BHT) on post thaw bovine semen characteristics of bovine crossbred spermatozoa.
MATERIAL AND METHODS
Two crossbred bulls (1/2 HF x 1/2 J) of aged 4-6 years weighing 450-500 Kg, reared at Semen Production Centre, Department of Veterinary Gynaecology & Obstetrics, College of Veterinary and Animal Sciences, G. B. Pant University of Agriculture and Technology, Pantnagar, Uttarakhand-263145 were used for experiment. The bulls were kept under identical feeding and management conditions.
Total eighteen (18) ejaculates from each bull were collected by Artificial Vagina method during morning hours. Eighteen (18) ejaculates from each bull were frozen separately after dilution in Tris extender with or without BHT. The fresh semen samples were evaluated for volume, pH, mass motility and sperm concentration. Ejaculates were selected on the basis of mass activity and individual motility. Ejaculates that had mass activity of 3.0 + (0-5.0 scale) and a progressive motility of 70% or more were selected for further extension and freezing (Salisbury and Vandemark, 1978).
BHT (275.44 mg) (SRL, India) was dissolved in 25 ml of ethanol making a BHT concentration of 0.05 mole/ml. 0.05 and 0.1ml of 0.05 mole/ml BHT was taken in the two glass test tubes and kept at 370C. Ethanol containing BHT was evaporated at resulting in sticking of BHT in the inner wall of the test tubes. The semen was extended with glycerolated egg yolk tris (EYT). In each BHT containing test tubes 5 ml of extended semen was added to make concentration @ 0.5 mM BHT (T1) and 1.0 mM BHT (T2). Tubes were kept at 370C for 5 min to allow uptake of BHT by spermatozoa. Simultaneously, the same amount of extended semen was kept in another test tube (without BHT) and considered as control.
Semen was filled in the 0.25 ml French mini straws at room temperature (370C) with the help of straw filling nozzle immediately after dilution. Polyvinyl alcohol powder was used to seal after creating air space with the help of air bubbler comb in each straw. Straws were wiped, dried, sealed and transferred to a refrigerator for equilibration at 4-50C for 4 hours. The samples were evaluated before deep freezing. After equilibration, semen samples were frozen using horizontal liquid nitrogen vapor freezing technique in a thermo cool box. The straws were maintained at a distance of 4 cm above the liquid nitrogen for 15 minutes to allow vapor freezing and transferred into goblets placed in the thermo cool box placed in the cryopreservation containers. After 24 hours of cryopreservation, the straws were thawed at 370C for 1 minute and evaluated for acrosomal integrity.
Semen was evaluated for progressive motility, viability and membrane integrity at three Stages i.e. after semen dilution and addition of BHT (0, 0.5 and 1.0 mM) (Stage I), after equilibration at 4-50C for 4 hours (Stage II) and after 24 hours of cryopreservation or post thaw (Stage III). After 24 h of cryopreservation, the straws were thawed at 37°C for 45 s. After thawing the semen was evaluated for progressive motility, sperm viability and sperm membrane integrity (HOST). Progressive motility was observed on a thermostatically regulated Stage, whereas, the sperm membrane integrity was evaluated by exposing the sperms to hypo-osmotic solution (150 mOsmol/L) (Jeyendran et al., 1984). Sperm viability was observed by Eosin-Nigrosin staining. Data was analyzed statistically (Snedecor and Cochran, 1989) by using SPSS computer package.
RESULTS AND DISCUSSION
Decrease in percentage individual motility from Stage I to Stage II was 8.44%, 8.28% and 9.36% (Table 1) in T1, T2 and control respectively, indicating lowest decrease in group T2. Reduction in individual motility from Stage II to Stage III was 13.78, 12.7 and 14.17 percent and from Stage I to Stage III, it was 21.78, 21.19 and 22.97 percent in T1, T2 and control, respectively (Table 1) (Figure 3). Difference in individual motility from Stage I to II and III indicated less decrease in motility in T2 and T1 than control though it was non-significant. Post thaw progressive motility of T2 and T1 was 7.05 and 3.25 percent more than control.
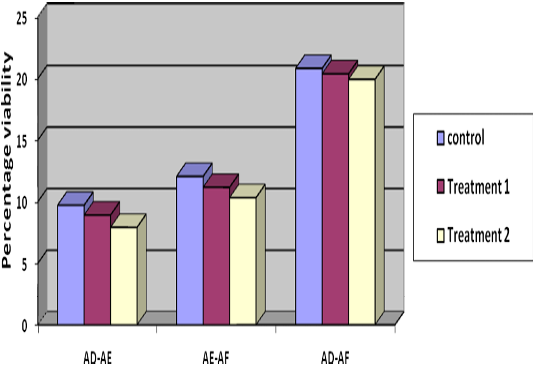
Decrease in percentage viability from Stage I to Stage II was 8.92%, 7.92% and 9.72% (Table 2) in T1, T2 and control respectively, indicating lowest decrease in group T2. Decrease in viability was 11.17, 10.33 and 12.06 percent from Stage II to Stage III and 20.39, 19.94 and 20.83 percent from Stage I to Stage III in T1, T2 and control group, respectively (Table 2) (Figure 2), indicated lesser loss in viability in group T2.
Percentage decrease in membrane stable spermatozoa from Stage I to Stage II was 7.67, 7.39 and 7.75 in T1, T2 and control groups, respectively (Table 3) which showed minimum loss of membrane stable spermatozoa in T2. Decrease in percentage membrane stable spermatozoa from II to Stage III was 9.86, 9.78 and 10.28 and from Stage I to Stage III was 17.78, 17.56 and 18.03 percent in T1, T2 and control respectively (Table 3) (Figure 1), indicated lowest loss in membrane stable spermatozoa in group T2.
Table 1: Difference of per cent progressive motility at three stages i.e. stage I, stage II and stage III (mean±SE, n= 36)
|
Group |
AD-AE |
AE-AF |
AD-AF |
|
Treatment 1 |
8.44±1.78C |
13.78±2.65B |
21.78±2.29A |
|
Treatment 2 |
8.28±1.4C |
12.75±2.5B |
21.19±2.2A |
|
Control |
9.36±1.99C |
14.17±2.74B |
22.97±2.14A |
Means bearing different superscript letters (A, B, C) in a column differ significantly (p<0.05)
n = No of samples, AD= after dilution, AE= after equilibration, AF= after freezing, AD-AE= difference of progressive motility between dilution to equilibration stage
Table 2: Difference of per cent viability of three stages (mean±SE, n= 36)
|
Group |
AD-AE |
AE-AF |
AD-AF |
|
Treatment 1 |
8.92±1.5B |
11.17±2.44B |
20.39±2.45A |
|
Treatment 2 |
7.92±1.27B |
10.33±2.53B |
19.94±2.60A |
|
Control |
9.72±1.75B |
12.06±2.74B |
20.83±2.59A |
Means bearing different superscript letters (A, B, C) in a column differ significantly (p<0.05)
n = No of samples, AD= after dilution, AE= after equilibration, AF= after freezing, AD-AE= difference of viaility between dilution to equilibration stage
Table 3: Difference of per cent membrane stable (HOS reactive) spermatozoa between three stages (mean±SE, n= 36)
|
Group |
AD-AE |
AE-AF |
AD-AF |
|
Treatment 1 |
7.67±1.07C |
9.86±2.23B |
17.78±2.59A |
|
Treatment 2 |
7.39±1.11C |
9.78±2.49B |
17.56±2.31A |
|
Control |
7.75±1.28C |
10.28±2.31B |
18.03±2.70A |
Means bearing different superscript letters (A, B, C) in a column differ significantly (p<0.05)
n = No of samples, AD= after dilution, AE= after equilibration, AF= after freezing, AD-AE= difference of HOS reactive spermatozoa between dilution to equilibration stage
Sperm membrane integrity is the index of sperm function. Loss in the membrane permeability is a common limitation during freezing and thawing due to the per-oxidative damage caused by free radicals and dilution of antioxidant defense (Khalifa et al., 2008). Increasing the antioxidant defense can retain the membrane intactness and subsequent reduction in free radical mediated oxidative damage to the cells (Andreea and Stela, 2010).Progressive motility serves as the index of fertilization (Ball et al., 2010). Post thaw viability of the sperms is always a challen-
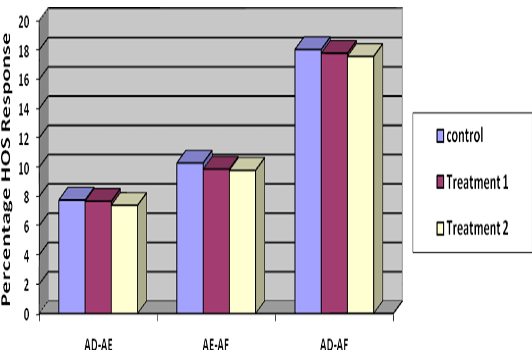
Figure 1: Difference of per cent membrane stable (HOS reactive) spermatozoa at three stages i.e. stage I, stage II and stage III
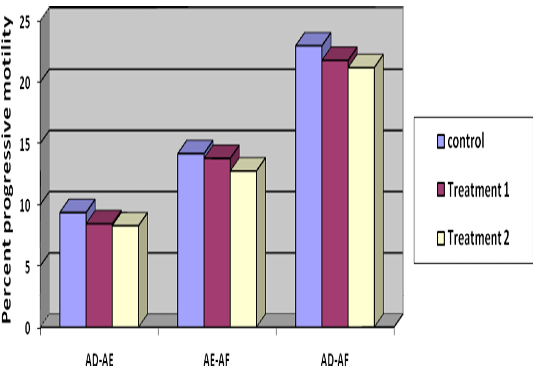
Figure 3: Difference of per cent progressive motility at three stages i.e. stage I, stage II and stage III
ging step because 30 to 40% viability of the sperms are decreased at the time of freezing and thawing, associated due to changes in the temperature, and ultra-low temperature maintained during cryopreservation (Watson, 2000). Sperm motility is nearly reduced by 50% after freezing and thawing in most of the species of the animal and may be a probable factor behind the reduction in the sperm quality (Watson, 2000). Thus, it is essential to maintain the sperm motility at a higher level after cryopreservation to achieve optimal fertility. To retain the motility and to minimize the free radical mediated oxidative stress, it is essential to improve the antioxidant defense of sperms opted for cryopreservation. It can be achieved by supplementing the extender with antioxidant additives (Ijaz et al., 2009).
Similarly, Patel et al (2015) also concluded that 1.0mM BHT improved post thaw motility of Holstein-Frisian bull spermatozoa in sodium citrate extender. Likewise in human, sperm viability and plasma membrane integrity was higher in 0.5mM BHT added semen (extended) (Jeyendran et al., 1984). Our results are contradictory with the study on Holstein-Friesian bull semen, where percentage of sperm with intact plasma membrane was higher in sodium citrate extender containing 0.5mM BHT (Shoae and Zamiri, 2008). Our results were also not same with the study that concluded that the supplementation of 0.5mM BHT in tris-citric acid extender improved the motility, plasma membrane integrity and viability of Sahiwal bull spermatozoa (Ansari et al., 2011).
It is believed that species and type of extender affect the sperm protection by BHT (Ball et al., 2001; Roca et al., 2004). Exotic bull semen has higher level of ROS as compared to zebu bull semen (Nichi et al., 2006). It is suggested more sperm motility in BHT supplemented semen is due to a decrease in oxidative stress and ROS production (Alvarez and Storey, 1983). The addition of BHT has improved the sperm quality by acting as an antioxidant thereby reducing the lipid peroxidation of the sperms (Patel et al., 2015). The highest (P < 0.05) motility, acrosomal integrity and hypo-osmotic swelling response of spermatozoa were achieved by addition of 1.0 and 2.0 mM BHT to semen extender to improve the semen quality of buffalo bulls (Ijaz et al., 2009). In the present study, reduction in motility from dilution to equilibration and post thaw was less in BHT added samples compared to control was probably due to its preventing role against ROS and reduction in membrane lipid per-oxidation. Furthermore, Post equilibration and post thaw higher sperm motility in supplemented groups is also indicative of protective action of BHT in cryopreserve sperms.
The maximum beneficial effects were observed by addition of 1.0mM BHT. Addition of BHT improved post thaw semen quality as revealed by higher progressive motility, viability and HOS response of spermatozoa though it was non-significant. Hence experiments to observe the effect of BHT on post thaw semen quality should be conducted at large scale.
ACKNOWLEDGMENTS
The authors are highly thankful to Vice Chancellor, Dean, College of Veterinary and Animal Sciences and Director Experiment Station of G.B Pant University of Agriculture and Technology, Pantnagar-263145, Uttarakhand, India for providing necessary facilities and financial support for carrying out this research.
Conflict of Interest
There is no conflict of interest.
Authors Contribution
All the authors have contributed in terms of giving their technical knowledge to frame the article
REFERENCES