Advances in Animal and Veterinary Sciences
Research Article
Prevalence and Associated Factors with Bovine Viral Diarrhoea Virus Antibodies in the Bulk Tank Milk of Small Scale Dairy Herds in Central Myanmar
Yi Mon Aye1, Min Aung2*, Win Ohnmar Kyaw2, Tint Naing3, Saw Po Po2
1Livestock Breeding and Veterinary Department, Kyaukse Township, Mandalay Region, Myanmar; 2Departments of Medicine, University of Veterinary Science, Yezin, Nay Pyi Taw, 15013, Myanmar; 3Crown Veterinary and Medical Resources, Yangon, Myanmar.
Abstract | This study was designed to determine the prevalence and to evaluate associated factors with bovine viral diarrhoea virus (BVDV) antibodies in the bulk tank milk of small scale dairy herds in central Myanmar. A cross sectional study was conducted from September to December in 2016 in four townships in central Myanmar. Bulk tank milk (BTM) samples were collected from 381 small scale dairy herds in the study area and analyzed for the presence of BVDV total antibody using commercial ELISA test kits. Several factors such as location of herd, herd size, number of lactating cows, calves and pregnant cows in the herd, and breeding practice were categorized and Pearson Chi-square test was used to determine the association between the factors and BVDV antibodies positivity. The prevalence of BVDV antibodies in the BTM of small scale dairy herds was 2.10%. The location of the herd, herd size, number of lactating cows, calves and pregnant cows in the herd were found to be significantly associated with the prevalence of BVDV antibodies, while breeding practice of the herd was not associated. Meikhtila region showed the highest prevalence (9.62%) of BVDV antibodies among studied locations. Specifically, in Meikhtila region, the dairy herd with more than 5 heads of cattle with the use of bull as the breeding practice had the greater potential of prevalence (25.00%) in comparison to that of other herd sizes and locations. Taken together, the prevalence of BVDV antibodies in the BTM of small scale dairy herds in central Myanmar was low it was influenced by factors such as location, herd size, number of lactating cows, calves and pregnant cows in the herd.
Keywords | Bovine viral diarrhea virus antibodies, Bulk tank milk, Prevalence, Small scale dairy herds, Myanmar
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 07, 2017; Accepted | July 10, 2017; Published | August 02, 2017
*Correspondence | Dr Min Aung, Department of Medicine, University of Veterinary Science, Nay Pyi Taw, Myanmar; Email: minaung.uvs@gmail.com
Citation | Aye YM, Aung M, Kyaw WO, Naing T, Po SP (2017). Prevalence and associated factors with bovine viral diarrhoea virus antibodies in the bulk tank milk of small scale dairy herds in central Myanmar. Adv. Anim. Vet. Sci. 5(8): 316-323.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.8.316.323
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Aye et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bovine viral diarrhoea (BVD), a widespread Pestivirus infection, causes serious clinical disease in cattle (Carlsson, 1991). BVD infections impact dairy industry directly through losses in production, and indirectly through market-related losses (Gunn et al., 2005). The economic losses are mainly associated with abortion, still births, congenital abnormalities, increased neonatal mortality, prenatal and postnatal growth retardation and persistently infected (PI) calves (Suddharshana et al., 1999). Moreover, the disease causes reduced milk production, poor reproductive performance, delayed growth, increased susceptibility to other diseases, early culling and increased mortality among stock (Houe, 2003).
An epidemiological study examining the effects of BVDV infection on the general health of cattle herds showed that BVDV associated with increased risk of clinical mastitis, retained placenta, oestrus stimulating treatments and longer calving intervals (Niskanen et al., 1995). BVDV is thought to be present in most cattle-raising countries and 60-90% of adult animals are seropositive (Sakhaee et al., 2009). The prevalence of BVDV infection differs between different countries and even between different provinces within a single country; this may be related to the differences in management, environmental variation, size of herds, and existence of persistently infected (PI) animals in these herds (Houe, 1999; Hemmatzadeh et al., 2001). Several factors may contribute to cause difference of seroprevalence of BVDV such as immuno-suppressive stress condition, dehydration, early weaning, control of environmental factors, low or high temperatures, and set up of biosecurity (Luzzago et al., 2010; Brodersen, 2010). Risk factor analysis suggested that routine vaccination for BVD, suspicion of BVD, housing of pregnant cows with calves, total number of cows and the proportion of cows were associated with increased BVDV antibodies in bulk milk (Humphry et al., 2012). Moreover, the highest prevalence was recorded in cows, followed by calves and heifers (Bedekovic et al., 2013) and the probability of any individual animal being infected or transmitting disease to susceptible cattle is strongly influenced by factors such as age, production type, and on-farm management practices (Carslake et al., 2011).
In Myanmar, it is still striving to develop the livestock production sectors, including dairy production, in which small scale dairy farming is playing a vital role. In the development of dairy production, the infectious diseases are one of the main determinants that can cause negative impacts on dairy production system. Thus, determination of prevalence and prevention of infectious diseases in dairy herds is very crucial. Among infectious diseases, BVDV is observed in most cattle-raising countries. Subsequently, many reports can be found in the literature regarding the prevalence of BVDV infection in countries around the world. However, there is no scientific report on the prevalence of BVDV infection in dairy herds in Myanmar. Prevalence of BVDV can be determined by identifying respective antibody in the serum or the milk. Antibody concentrations in BTM are indicative of the prevalence of immune cows in the milking herd (Beaudeau et al., 2001; Eiras et al., 2012).Testing bulk tank milk (BTM) can be a valuable, efficient and cost-effective method for determining herd immunity. Determining a threshold for the detection of herds containing active BVD infection by testing bulk milk is a novel use for an antibody ELISA kit and provides more practical, relevant test results (Lanyon et al., 2014). Thus, the study aimed to determine the prevalence and associated factors with BVDV antibodies in the bulk tank milk of small scale dairy herds in central Myanmar.
Materials and methods
Study Area and Target Dairy Herds
This study was conducted in central Myanmar, covering Patheingyi, Kyaukse, Tada-U and Meikhtila townships. The target destination was small scale dairy herds of cross-bred Holstein Friesian dairy cows. A cross sectional study was conducted from September to December, 2016 to collect the bulk tank milk (BTM) samples.
Sample Size Estimation and Btm Samples Collection
For the estimation of sample size, the formula of Daniel (1999) was used, which was as followed;
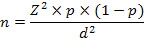
Where,
n = Sample size
Z = Confidence level
p = Prevalence
d = Confidence interval
For calculation, 50% prevalence and 95% confident level were selected. The total required sample was determined as 384 samples; however we used only 381 samples in this study because 3 samples were failure in the process of laboratory analysis. Prior to collection of BTM, the milk container tubes were autoclaved and about 10ml of BTM was collected into 15 ml centrifuge tubes from each small scale dairy herd. The samples were stored in a chiller with the ice packs and brought to the veterinary diagnostic laboratory (Mandalay) for further analysis.
Sample Collection and Collection of Herd Data
The bulk tank milk (BTM) samples were collected from the target herds from September to December in 2016. At the time of sample collection, a questionnaire interview was conducted, covering Herd location (township), Herd size (>5 heads or ≤5 heads), Number of lactating cows in the herd (>3 or ≤3), number of calves in the herd (>2 or ≤2), Number of pregnant cows in the herd (>2 or ≤2) and Breeding practice (Artificial Insemination or natural mating with bulls).
Detection of BVDV Antibody with ELISA
Prior to the BVDV antibody test, the BTM samples were centrifuged for 15 minutes at 2000g to separate the skimmed milk from the fat layer. The antibodies could be detected in the skimmed milk, which were under the fat layer. After that, the ELISA procedure to detect the antibody against BVDV was carefully conducted according to the protocol provided by the manufacturer, IDEXX Laboratories.
Calculation and Interpretation of Sample to Positive Ratio
The presence and absence of BVDV antibodies in the sample is determined by sample to positive ratio (S/P ratio) for each sample. The formula [S/P = (Sample-Negative control)/(Positive control-Negative control)] was used to calculate the S/P ratio. The validity criteria for negative control was ≤0.250 and positive control-negative control was ≥ 0.150. If the value of S/P ratio was less than 0.20, it was determined as negative and the S/P ratio greater than or equal 0.20 was determined as positive.
Statistical analysis
After analysis of the samples,the results were entered and validated in Microsoft Excel program. After that the data was imported into SPSS (version 16) software for statistical analysis. Factors associated with BVDV were analysed by using Pearson Chi-square test and P<0.05 is considered as the significant level.
Results
The prevalence and associated factors with BVDV antibodies in the BTM of small scale dairy herds are presented in Table 1. The factors are categorized as the location, herd size, number of lactating cows, calves and pregnant cows and breeding practice. Eight samples were found positive for BVDV antibody out of the total 381 samples indicated that the prevalence of BVDV antibodies in the BTM of small scale dairy herds was 2.10%. The factors such as location of herds, herd size, number of lactating cows, calves and pregnant cows were significantly associated (P<0.05) with the prevalence of BVDV antibodies in the BTM, while breeding practice was not associated with it. Meikhtila region had the highest potential of prevalence (9.62%) of BVDV antibodies among the locations. The dairy herds with greater than 5 cattle (5.10%) or 3 lactating cows (6.25%) or 2 calves (5.49%) or 2 pregnant cows (10.00%) also had higher potential of prevalence of BVDV antibodies in BTM in comparison to less than or equal 5 cattle (1.06%) or 3 lactating cows (1.00%) or 2 calves (0.78%) or 2 pregnant cows (1.11%), respectively.
The prevalence of BVDV antibodies in BTM based on number of lactating cows, calves, herd size and locations are shown in Table 2 and 3, respectively. According to the results, the combination of herd size (>5), number of lactating cows (>3) or calves (>2), and locations was related (P<0.05) with the prevalence of BVDV antibodies in the BTM. The small scale dairy herds (herd size>5)in Meikhtila region with number of lactating cows(>3) or calves (>2) had the highest potential of prevalence (26.67% and 23.53%, respectively) of BVDV antibodies in BTM in comparison to other regions and herd size and number of lactating cows or calves.
The prevalence of BVDV antibodies in BTM based on number of pregnant cows, herd size and locations are described in Table 4. The combinations of herd size, number of pregnant cows and locations were not associated (P>0.05) with the prevalence of BVDV antibodies in BTM, while they were as an individual factor significantly associated (P<0.05) with the prevalence of BVDV antibodies in the BTM (Table 1).
The prevalence of BVDV antibodies in BTM based on breeding practices, herd size and locations are defined in Table 5. No significant relationship (P>0.05) between breeding practice and prevalence of BVDV antibodies was observed (Table 1), however the combination of herd size (>5), breeding practice (Bull) and locations were related (P<0.05) with the prevalence of BVDV antibodies in BTM. The small scale dairy herd (herd size>5) in Meikhtila region with using of bull as the breeding practice had the greater potential of prevalence (25.00%).
Discussion
The prevalence of BVDV antibodies in the BTM of small scale dairy herds in central Myanmar was 2.10% (8/381), which is lower than the 73%prevalence observed in BTM of dairy herds in northern and north-eastern Thailand (Kampa et al., 2004) and practically similar to the 3.1% prevalence detected in the BTM of dairy herds in Finland (Niskanen, 1993). On the other hand, seroprevalence of BVDV antibodies in the neighbouring countries are 24% in Qinghai, China (Gong et al., 2014), 43% in Western China (Zhong et al., 2011), 33.2% in Selangor, Malaysia (Daves et al., 2016), 16.85% in Pakistan (Gohar et al., 2013) and 17.1% in India (Behera et al., 2011). Thus, it could be assumed that the prevalence of BVDV antibodies in Myanmar is less than that of those countries. The reason for this finding might be due to lack of BVDVvaccine usage in Myanmar. Durham and Hassard (1990) reported that the prevalence of seropositive cattle was influenced by the use of vaccine.
Cautions should be taken in comparing prevalence data of BTM and serological samples. There seems to be divergent views. Niskanen reported that the level of antibodies against BVDV in bulk milk relates to the prevalence of BVDV seropositive lactating cows in the dairy herd (Niskanen, 1993). However, two other researchers (Houe, 1994; Beaudeau et al., 2001) reported that the antibody titers from bulk milk are loosely correlated with true disease status or with the number of antibody positive animals. Antibody levels in bulk milk can only contribute incomplete evidence of herd status because bulk milk scores give an uncontrolled, pooled summary of the antibody levels of the contributing animals. If antibody positive in BTM are not detected, the herd is classified as BVD negative, but hi-
Table 1: Prevalence and associated factors with BVDV antibodies in BTM of small scale dairy herds
|
Categories |
No. of farms |
No. of positive farm (%) |
Chi square value |
P value |
|
|
Overall* |
381 |
8 (2.10%) |
- |
- |
|
|
Location of herds |
|||||
|
Patheingyi |
48 |
0 (0.00%) |
18.394 |
0.0004 |
|
|
Kyaukse |
20 |
1 (5.00%) |
|||
|
Tada-U |
261 |
2 (0.77%) |
|||
|
Meikhtila |
52 |
5 (9.62%) |
|||
|
Herd size |
|||||
|
>5 |
98 |
5 (5.10%) |
5.785 |
0.0162 |
|
|
≤5 |
283 |
3 (1.06%) |
|||
|
No. of lactating cows |
|||||
|
>3 |
80 |
5 (6.25%) |
8.485 |
0.0036 |
|
|
≤3 |
301 |
3 (1.00%) |
|||
|
No. of calves |
|||||
|
>2 |
91 |
5 (5.49%) |
7.584 |
0.0059 |
|
|
≤2 |
257 |
2 (0.78%) |
|||
|
No. of pregnant cows |
|||||
|
>2 |
20 |
2 (10.00%) |
7.256 |
0.0070 |
|
|
≤2 |
180 |
2 (1.11%) |
|||
|
Breeding practice |
|||||
|
AI |
53 |
1 (1.89%) |
0.014 |
0.9070 |
|
|
Bull |
328 |
7 (2.13%) |
|||
BVDV: bovine viral diarrhea virus, BTM: bulk tank milk, AI: artificial insemination
* The 15 out of 381 samples showed the negative results; however the titre of BVDV antibodies in BTM were slightly high.
Table 2: Prevalence of BVDV antibodies in BTM based on number of lactating cows, herd size and locations
|
Herd size |
No. of Lactating cows |
Location |
No. of farm |
No. of positive farm (%) |
Chi square value |
P value |
|
>5 |
>3 |
Patheingyi |
6 |
0 (0.00%) |
12.825 |
0.005 |
|
Kyaukse |
8 |
1 (12.50%) |
||||
|
Tada-U |
42 |
0 (0.00%) |
||||
|
Meikhtila |
15 |
4 (26.67%) |
||||
|
≤3 |
Patheingyi |
5 |
0 (0.00%) |
- |
- |
|
|
Kyaukse |
4 |
0 (0.00%) |
||||
|
Tada-U |
16 |
0 (0.00%) |
||||
|
Meikhtila |
2 |
0 (0.00%) |
||||
|
≤5 |
>3 |
Patheingyi |
1 |
0 (0.00%) |
- |
- |
|
Kyaukse |
1 |
0 (0.00%) |
||||
|
Tada-U |
6 |
0 (0.00%) |
||||
|
Meikhtila |
1 |
0 (0.00%) |
||||
|
≤3 |
Patheingyi |
36 |
0 (0.00%) |
1.558 |
0.669 |
|
|
Kyaukse |
7 |
0 (0.00%) |
||||
|
Tada-U |
197 |
2 (0.67%) |
||||
|
Meikhtila |
34 |
1 (2.94%) |
BVDV: bovine viral diarrhea virus, BTM: bulk tank milk
Table 3: Prevalence of BVDV antibodies in BTM based on number of calves, herd size and locations
|
Herd size |
No. of calves |
Location |
No. of farm |
No. of positive farm (%) |
Chi square value |
P value |
|
>5 |
>2 |
Patheingyi |
9 |
0 (0.00%) |
13.267 |
0.004 |
|
Kyaukse |
9 |
1 (11.11%) |
||||
|
Tada-U |
48 |
0 (0.00%) |
||||
|
Meikhtila |
17 |
4 (23.53%) |
||||
|
≤2 |
Patheingyi |
2 |
0 (0.00%) |
- |
- |
|
|
Kyaukse |
3 |
0 (0.00%) |
||||
|
Tada-U |
9 |
0 (0.00%) |
||||
|
Meikhtila |
- |
- |
||||
|
≤5 |
>2 |
Patheingyi |
2 |
0 (0.00%) |
- |
- |
|
Kyaukse |
- |
- |
||||
|
Tada-U |
3 |
0 (0.00%) |
||||
|
Meikhtila |
3 |
0 (0.00%) |
||||
|
≤2 |
Patheingyi |
33 |
0 (0.00%) |
2.652 |
0.448 |
|
|
Kyaukse |
8 |
0 (0.00%) |
||||
|
Tada-U |
171 |
1 (0.58%) |
||||
|
Meikhtila |
31 |
1 (3.23%) |
BVDV: bovine viral diarrhea virus, BTM: bulk tank milk
Table 4: Prevalence of BVDV antibodies in BTM based on number of pregnant cows, herd size and locations
|
Herd size |
No. of pregnant cows |
Location |
No. of farm |
No. of positive farm (%) |
Chi square value |
P value |
|
>5 |
>2 |
Patheingyi |
2 |
0 (0.00%) |
3.754 |
0.289 |
|
Kyaukse |
8 |
1 (12.50%) |
||||
|
Tada-U |
5 |
0 (0.00%) |
||||
|
Meikhtila |
2 |
1 (50.00%) |
||||
|
≤2 |
Patheingyi |
6 |
0 (0.00%) |
5.085 |
0.166 |
|
|
Kyaukse |
4 |
0 (0.00%) |
||||
|
Tada-U |
40 |
0 (0.00%) |
||||
|
Meikhtila |
10 |
1 (10.00%) |
||||
|
≤5 |
>2 |
Patheingyi |
- |
- |
- |
- |
|
Kyaukse |
- |
- |
||||
|
Tada-U |
3 |
0 (0.00%) |
||||
|
Meikhtila |
- |
- |
||||
|
≤2 |
Patheingyi |
7 |
0 (0.00%) |
0.252 |
0.969 |
|
|
Kyaukse |
5 |
0 (0.00%) |
||||
|
Tada-U |
96 |
1 (1.04%) |
||||
|
Meikhtila |
12 |
0 (0.00%) |
BVDV: bovine viral diarrhea virus, BTM: bulk tank milk
gh bulk milk titers should be taken into consideration (Houe, 1999). In this study, 15 out of 381 samples showed the negative results; however the titre of BVDV antibodies in BTM were slightly high. The S/P ratio of those samples ranged from 0.101 to 0.190, whereas the S/P ratio ≥0.20 was determined as the positive. These results indicated that although BVDV antibodies in BTM were negative, the antibodies in individual animals might be positive. Thus, the authors recommend seroprevalence studies of BVDV in the dairy herds of Myanmar.
The reasons for the highest prevalence in Meikhtila region among the locations might be due to different management systems, environmental condition and cattle move
Table 5: Prevalence of BVDV antibodies in BTM based on breeding practices, herd size and locations
|
Herd size |
Breeding practice |
Location |
No. of farm |
No. of positive farm (%) |
Chi square value |
P value |
|
>5 |
AI |
Patheingyi |
0 |
0 (0.00%) |
0.110 |
0.740 |
|
Kyaukse |
10 |
1 (10.00%) |
||||
|
Tada-U |
0 |
0 (0.00%) |
||||
|
Meikhtila |
1 |
0 (0.00%) |
||||
|
Bull |
Patheingyi |
11 |
0 (0.00%) |
18.605 |
0.001 |
|
|
Kyaukse |
2 |
0 (0.00%) |
||||
|
Tada-U |
58 |
0 (0.00%) |
||||
|
Meikhtila |
16 |
4 (25.00%) |
||||
|
≤5 |
AI |
Patheingyi |
- |
- |
- |
- |
|
Kyaukse |
6 |
0 (0.00%) |
||||
|
Tada-U |
36 |
0 (0.00%) |
||||
|
Meikhtila |
- |
- |
||||
|
Bull |
Patheingyi |
37 |
0 (0.00%) |
1.235 |
0.745 |
|
|
Kyaukse |
2 |
0 (0.00%) |
||||
|
Tada-U |
167 |
2 (1.20%) |
||||
|
Meikhtila |
35 |
1 (2.86%) |
BVDV: bovine viral diarrhea virus, BTM: bulk tank milk, AI: artificial insemination
ment. Meikhtila region has live cattle markets, resulting in increased exposure of infection to new animals. Identifying cattle movements that are associated with the greatest risk of infectious disease transmission has the important implications for refining future epidemiological models and disease control strategies (Gates et al., 2014). Trade is known as one of the epidemiological determinants for the introduction and spread of BVDV in cattle herds (Stahl and Alenius, 2012; Houe 1999; Hemmatzadeh et al., 2001) reported that the prevalence of BVDV infection differs between different countries and different provinces within a country; this may be related to the differences in management, environmental variation, size of herds, and existence of persistently infected (PI) animals in these herds.
Another possible explanation for higher prevalence in Meikhtila region is due to the use of common milking man for most of the small scale dairy herds. The milking men are generally used for the collection of milk from small scale dairy herds in Myanmar. According to the personal communication data from this study, only a very few milking men were used to collect milk in Meikhtila region, while more milking men were used in other regions. (Niskanen and Lindberg 2003; Almeida et al., 2013) reported that the significant association between BVDV positivity and lactation might be due to the higher risk of getting infection from the workers who milk the cows and the virus can easily be transmitted through fomites such as contaminated cloth or equipment which are used during the milking process.
In this study, the dairy herd with herd size (>5) or number of lactating cows (>3) or number of calves (>2) or number of pregnancy cows (>2) had the highest potential for the prevalence of BVDV antibodies in the BTM. More specifically, large herd size and number of lactating cows and calves in Meikhtila had the highest association with BVDV prevalence in comparison to other various herd conditions and locations. These findings are consistent with the previous published reports (Talafha et al., 2009; Bedekovic et al., 2013; Kulangara et al., 2015). The influence of herd size, mean distance to neighbouring herds, and sharing of common pasture are proven as important risks factors in BVD epidemiology (Alban et al., 2001).
Although, in general, the breeding practices and prevalence of BVDV antibodies were found to be not associated in this study, the highest prevalence of BVDV antibodies was observed in the larger dairy herds (herd size>5) of Meikhtila region, in which bull was used as the breeding practice. This finding agrees with the report of Marques et al. (2016), high animal density, weaning age ≤60 days, exchange of animals,calf mortality >5% and use of natural breeding and artificial insemination were identified by the author as the associated risk factors of BVDV infection.
Conclusion
The prevalence of BVDV antibodies in the BTM of small scale dairy herds in the central Myanmar was low. The prevalence was influenced by the factors such as location of the head, herd size, number of lactating cows, calves and pregnant cows in the herd. Moreover, the dairy herds (herd size>5) in Meikhtila region with the increased number of lactating cows (>3) or calves (2>) or breeding practice (Bull) had the highest prevalence of BVDV antibodies in the BTM of the small scale dairy herds in central Myanmar. The authors recommend to determine the seroprevalence of BVDV in the dairy herds of Myanmar.
Acknowledgement
We would like to thank all laboratory staffs from the department of medicine, University of Veterinary Science and Veterinary Diagnostic Laboratory, Mandalay for their helps in the process of detection of BVDV antibodies in the bulk tank milk.
Conflict of Interest
There is no conflict of interest.
AUTHOR’S CONTRIBUTION
YMA mainly carried out sample collection and analysis. MA, WOMK, TN and SPP designed the experiment. MA performed data analysis and interpretation. YMA drafted the manuscript and MA, WOMK, TN and SPP completed the critical revision of the article. All authors read and approved the final version of manuscript.
References





