Advances in Animal and Veterinary Sciences
Research Article
Serosurveillance and Molecular Detection of Bovine Herpesvirus-1(BHV-1) in Cattle and Buffaloes in Baghdad
Shakir Frayyeh Nezzal¹, Ibtesam Qasim Hassan¹, Ruqaya Mustafa Ali²
1Veterinary Medicine College / Department of Internal and Prevention Veterinary Medicine/ Baghdad University; 2Ministry of Agriculture-Central Veterinary Laboratory, Baghdad, Iraq.
Abstract | Infectious bovine rhinotracheitis virus (IBRV)-caused clinical menfestations limits animal trades and cause major economic losses in large ruminants. However, the symptoms are mainly not life-threatening. Assessment of true prevalence of IBR would be phenomenal in devising control strateges against the disease.This study aim to address the field clinical observations of IBRusing serological and molecular approaches in cattle and buffaloes in differnet Baghdad governorates.A total of 368 animals (243 cattle and 125 buffaloes) were sampled during July – December 2016 and screened for the presence of antibodies agianstBHV-I using indirect ELISA. Atotal of 131 (35.59%) samples were positive by indirect ELISA and the positivity of80 (32.92%) samples from cattle and 51 (40.80%) samples from buffaloes were determined. The clinical symptoms which accompanied the positive casesinclude respiratory signs, ocular signs, abortion, diarrhea and fever.In cattle, highest positive cases (n=16, 20%) were in animals whichwere showing respiratory signs. Amongest the positive cases, a lowest seropostivity (n=1, 1.25%) was recorded in animals that were also positive for clinical signs such as abortion and mastitis with or without fever. In buffaloes, the highest positive cases (n=15, 29.41) were in animals that shown respiratory signs accompanied with fever. The lowest positive cases in animals that were showing clicnical signs including ocular signs, respiratory and ocular and abortion accompanied with ocular plus fever.Among samples that were serologically positive, a total of50 different samples (whole blood, nasal swabs and abomasal contents of aborted fetuses) from both cattle and buffaloes were subjected to conventional PCR for molecular detection. Out of testedsamples, 37 (74.00%) samples were positive for the presence of viral genetic material with expected product size of 175bp. Presence of BHV1 was highest in abomasal contents (87.50%) followed by whole blood (83.33%) and nasal swabs (55.55%). Taken together, the findings of the study demonstrate the prevalence of the disease in the country and warrant devising and planning control measure to prevent economic lossess to livestock industry.
Keywords | IBR, Infectious bovine rhinotracheitis, BHV1, ELISA, PCR, Baghdad.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 12, 2017; Accepted | July 02, 2017; Published | July 19, 2017
*Correspondence | Shakir Frayyeh Nezzal, Veterinary Medicine College / Department of Internal and Prevention Veterinary Medicine/ Baghdad University, Baghdad, Iraq; Email: shakirnezal@gmail.com
Citation | Nezzal SF, Hassan IQ, Ali RM (2017). Serosurveillance and molecular detection of bovine herpesvirus-1(bhv-1) in cattle and buffaloes in Baghdad. Adv. Anim. Vet. Sci. 5(7): 283-288.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.7.283.288
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Nezzal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Infectious bovine rhinotracheitis known as “red-nose”, necrotic rhinitis or dust pneumonia (Majumder et al., 2015) and is considered a highly contagious infectious disease. It is caused by bovine herpesvirus1 (BHV1) which belongs to the genus Varicellovirus in the family of Herpesviridae, subfamily Alphaherpesvirinae and order Herpesvirales (Velzen, 2013). The virus is classified into three subtypes; 1.1, 1.2a and 1.2b (Radostits et al., 2007; Nandi et al., 2009). It has a worldwide distribution and causes major economic losses in cattle production, especially in the dairy industry (Nardelli et al., 2008). The disease has several clinical manifestations, including respiratory tract infection, keratoconjunctivitis, infectious pustular vulvovaginitis (IPV), infectious pustular balanoposthitis (IPB), abortion, encephalitis, enteritis as well as systemic infection. The encephalitis and enteritis are predominantly presents in young animals and are usually fatal (Nardelli et al., 2008; Sasani et al., 2013).
There are different methods for the diagnosis of BHV1 such as cell culture-based identification, serological assesemnt of antibodies or molecular methods for the detection of viral genome. Indirect ELISA is being widely used for BHV1 antibodies in cattle population in different regions of the world (Saravanajayam et al., 2015). Polymerase chain reaction is more sensitive, specific and relatively rapid in comparisonto virus isolation (results are available within 12h) (Dehkordi et al., 2013). This molecular technique can be used to detect BHV1 glycoprotein C in the aborted fetus and semen of calves, cattle, and buffalo. Beside this target,gB, gD and thymidine kinase genes of BHV1 can also be targeted for molecular detection albietvariable sensitivities (Wang et al., 2008; Majumder et al., 2015). The virus can be detectedin the nasal swabsfor up to 14 days post-infection (Nandi et al., 2009). The abomasal contents of aborted fetuses is also considered a reliable choice for the detection of BHV1 detection (Dehkordi et al., 2013).
The first isolation of the virus in Iraq was demonstrated in 1976 (Mahmood, 1976). However, scarce information is available regarding the serosurveillance and molecular investigation of IBR in cattle and buffaloes in the country. This study was designed to detect antibodies against BHV1 by indirect ELISA and for the detection of BHV1 by PCR in cattle and buffaloesusing different types of samples. Additionally, to enlist clinical symptoms of IBRthat are more common in the field in Baghdad governorates.
Materials and Methods
Animals and Samples
Blood samples were collected from 368 IBR-suspected cattle and buffaloeswith different clinical signs in different locations of Baghdad.Blood (8-10 ml) samples were taken from the jugular vein by vacutainer tubes without anticoagulant, and held at room temperature for approximatelyhalf an hour at slant to allow the separation of blood serum. Samples were stored in the cool box with ice, then centrifuged at 2500 rmp for 10 minutes then kept at -20 °C untiluse for the detection of antibodies in indirect ELISA.
For detection of DNA, whole blood samples were collected from the jugular vein ina tube with anticoagulant before storeing in a cold place. Nasal swab samples were taken and were placed inside tubescontaining viral transport media. Abomasal contents of aborted fetus were taken and stored in freezer. Twenty four whole blood, 18 nasal swabs and 8 abomasal contents of aborted fetus were randomly taken from ELISA-seropositive animals. The samples of blood were taken as pointed by Fuchs et al. (1999); nasal swabs as pointed by Saha et al. (2010) and abomasal content of aborted fetus were taken as demonstrated by (Dehkordi et al., 2013).
Serological Assays
IBR detection kit (IDvet, France) (indirect ELISA) was used for the detection of antibody against BHV1 in cattle and buffaloes serum and was used according to manufacturer’s instructions. For the assay to be valid, the mean value of the positive control O.D. (OD pc) must be greater than 0.350 (OD pc > 0.350). In addition, the ratio of mean O.D values of the positive and negative must be greater than 3. Optical density (OD) was read at 450nm in a multi-well plate reader (EXL 800 Biotic, USA). The sensitivity and specificity of the kit were 98.00%. The serum samples with OD ≥ 0.60 were considered as positive cases. According to instructions, the calculation of result was based on special equation S/P%
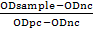
the results less than 50% were considered negative, the results greater or equal to 50% and less than 60% were considered doubtful and if the results greater or equal to 60% were considered positive.
Polymerase Chain Reaction (Pcr)
DNA was extracted using the commercially available DNA extraction kit (Qiagen, Germany), as indicated in the manufacturer’s protocol. The DNA concentration and purification was measured using scan-drop spectrophotometer (Analytikjena, Germany). Primer sequences were designed based on the sequence of the BHV-1 glycoprotein B (gB) gene as demonstrated by the (Chandranaik et al., 2010; Dehkordi et al., 2013). The PCR primers were synthesis by DNA Alfa Company/Canada(gB-F 5’ TGT GGA CCT AAA CCT CAC GGT 3’, gB-R 5’ GTA GTC GAG CAG ACC CGT GTC 3’). PCR Master Mix was prepared by using (GoTaq® Green Master Mix/Promega) according to instructions.Preparation and examination of agarose gel was performed as mentioned by Maniatis et al. (1982).The DNA products wereshownunder ultraviolet (UV) light after electrophoresis of 10 μL of the PCR products in a 2% agarose stained with ethidium bromide (0.3 mg⁄mL) using 100-bp molecular weight marker (Vivantis, Malaysia).
Data Analysis
The Statistical Analysis System- SAS (2012) program was used to evaluate different factors and parameters.
Results
Firstly, this study was approved by the ethical and research committee of veterinary medicine college/university of Baghdad. The suspected cattle and buffaloes were suffering from one or more clinical signs; abortion, respiratory signs, ocular symptoms, fever, mastitis and diarrhea (Table 1 and Figure 3). In 80 of positive serum samples in cattle, the highest positive (n=16, 20%) cases were in animals which have shown respiratory signs only. Abortionand mastitis with or without fever showed the lowest seropostivity (n=1, 1.25%) (Table 1 and Figure 1). In buffaloes, the highest positive cases (n=15, 29.41%) were in animals with respiratory signs accompanied with fever.The lowest positive cases (1, 1.96%) were in animals that shown ocular signs only, respiratory plus ocular and abortion accompanied with ocular plus fever (Table 1; Figure 2).

Figure 1 A diagram showing the percentages of various clinical signs of seropositive cases in cattle
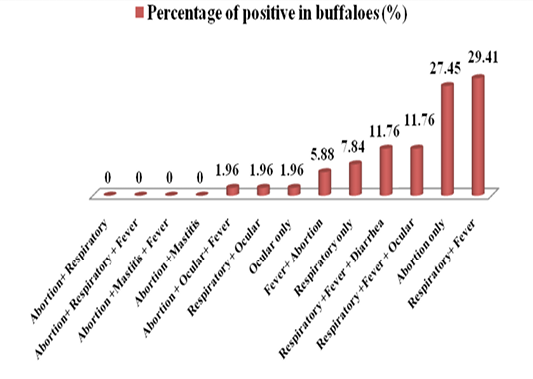
Figure 2: A diagram showing the percentages of various clinical signs of seropositive cases in buffaloes
Table 1: Percentages of positive clinical cases of IBR in cattle and buffaloes detected by indirect ELISA.
|
(P>) |
Chi-Square |
Numbers of positive cases in buffaloes (%) |
Numbers of positive cases in cattle (%) |
Clinical signs |
|
0.17 |
1.83 |
14(27.45) |
14(17.50) |
Abortion only |
|
0.72 |
0.12 |
3(5.88) |
6(7.50) |
Fever+ Abortion |
|
0.25 |
1.29 |
0(0.00) |
2(2.50) |
Abortion+ Respiratory |
|
0.06 |
3.31 |
0(0.00) |
5(6.25) |
Abortion+ Respiratory + Fever |
|
0.56 |
0.33 |
1(1.96) |
3(3.75) |
Abortion + Ocular+ Fever |
|
0.42 |
0.64 |
0(0.00) |
1(1.25) |
Abortion +Mastitis + Fever |
|
0.42 |
0.64 |
0(0.00) |
1(1.25) |
Abortion +Mastitis |
|
0.05 |
3.55* |
4(7.84) |
16(20.00) |
Respiratory only |
|
0.05 |
3.95* |
15(29.41) |
12(15.00) |
Respiratory+ Fever |
|
0.26 |
1.23 |
6(11.76) |
5(6.25) |
Respiratory +Fever + Diarrhea |
|
0.92 |
0.008 |
6(11.76) |
9(11.25) |
Respiratory +Fever + Ocular |
|
0.56 |
0.33 |
1(1.96) |
3(3.75) |
Respiratory + Ocular |
|
0.56 |
0.33 |
1(1.96) |
3(3.75) |
Ocular only |
|
51 |
80 |
Total |
||
|
(9.273 **) |
(8.409 **) |
Chi-Square |
0.00 Not noticed, ** (P<0.01), * with (P<0.05)
Statistically, there were no significant differences in positive percentages of clinical signs between cattle and buffaloes except in respiratory signs only and respiratory accompanied with fever (P<0.05). Also, there were significant differences in overall of positive percentages of clinical signs in cattle and buffaloes with probability of P<0.01.
After demonstration of seropositivities, a total of 50 different samples (24 whole blood, 18 nasal swabs and 8 abomasal contents of aborted fetuses from cattle and buffaloes) were taken randomly from sero-positive animals to detect BHV1 by conventional PCR assays (Table 2). The BHV1
genome was detected by targetting the gB-based primers with a 175 bp long amplicons (Figure 4). Results showed that 37 out of 50 seropositive animals (74.00%) were positive for the presence of BHV1. However,the percentage of positive samples among the different samples of whole blood, nasal swabs and abomasal contents of aborted fetus were 83.33%, 55.55% and 87.50% respectively.
Table 2: Molecular detection of IBR by PCR
|
Type of samples |
No. of samples |
No. of positive samples |
No. of percentages of positive samples (%) |
No. of negative samples |
No. of percentages of negative samples (%) |
Chi-Square |
|
Whole blood |
24 |
20 |
83.33 |
4 |
16.66 |
13.407 ** |
|
Nasal swabs |
18 |
10 |
55.55 |
8 |
44.44 |
4.315 * |
|
abomasal contents of Aborted fetus |
8 |
7 |
87.50 |
1 |
12.50 |
13.742 ** |
|
Total |
50 |
37 |
74.00 |
13 |
26.00 |
12.064 ** |
|
Chi-Square |
--- |
--- |
9.288 ** |
--- |
9.288 ** |
--- |

Figure 3: IBR-positive animals showed variant clinical signs (a- Aborted cow, b- Ocular signs, c and d- Respiratory signs)

Figure 4: Agarose gel electrophoresis image that shows the PCR product analysis of BHV 1 gB gene. Where M: DNA molecular weight ladder of 100bp, lane (2-11) positive field samples at 175 bp PCR product, lane 1 positive control and lane 12 negative control.
On statistical analysis, there were significant differences between positive and negative percentages in different type of samples with P<0.01 except between positive and negative nasal swabs was withP<0.05. As well as, there were significant differences among different types of samples with P<0.01.
Discussion
The presented data revealedthatthe vast majority of seropositive cases were constituted by mixed clinical signs. These results are comparable with the data of Rhaymah et al. (2012). There were also variations in the results of several studies, Ghazy et al. (2007) who revealed that percent of reproductive disorders was 78.2% in buffaloes. Whereas, in Mousl, Rhaymah et al. (2012) found that seropostivity of the percentage of positive sera of respiratory signs was 67.40%, abortion was 72.7% and ocular was 62.5% in cattle. Also high seropositive percentage (76.5%) with abortion in the cattle appeared in the results of Romero-Salas et al. (2013). While, Saravanajayam et al. (2015) found that the seropostivity of respiratory signs was 63.64%, while 70.89% with reproductive signs in cattle.
This considerable variation in the percentages of positive clinical signs in the present study may relate to natural infection, type of viral strain, age susceptibility and environmental factors (Radostits et al., 2007). Also, it may be due to the secondary bacterial infection, which leads to rise of severity of the disease and appearanceof different clinical signs in the same case which is supported Muylkens et al. (2007). This highlight the need to monitor both viral and bacterial infections simultaneously.
The percentages of positive samples by PCR in this study (Table 2) were relatively lower than the results obtained from the study of Elhassan et al. (2015) who have detected 87.70% positive cases for BHV1. Sarmah et al. (2015) found lower percentages than the present study with results of 53.84% whereas Rola et al. (2005) found almost the same percentage with the present study for nasal swabs (44%).
The lower virus DNA extracted from nasal swabs was probably because of the decline of concentration of virus excreted from respiratory routs and this concentration increases if the nasal swaps have been taken from suspected animals during stress period (Ranganatha et al., 2013). Besides that,the shedding of virus occurs between the third and sixth days (during the early acute stage of the disease) so that the nasal swabs should be taken early when the discharge is more serious rather than mucopurulent (Saha et al., 2010; OIE, 2016). The important fact to be mentioned here, the shedding of the virus may or may not be accompanied with clinical signs (Muylkens et al., 2007).
Conclusions
The present study concluded that different clinical forms are shown in cattle and buffaloes,which consequently lead to big economic losses.This study reported for the first time the diagnosis of BHV1 in buffaloes by PCR in Iraq. ELISA andPCRappeared to suitable and useful techniquesfor the diagnosis of IBR, where PCR is fast, reliable diagnostic method for detection of BHV1 in both cattle and buffaloes.
Acknowledgements
The authors thank anyone who offered any helping to complete this study.
Conflicting Interests
The authors declare that they have no conflicting interests.
Authors Contribution
Shakir Frayyeh Nezzal: The main researcher in completing the research. Ibtesam Qasim Hassan: Scientific Supervisor Ruqaya Mustafa Ali: Assist in completing the PCR test.
References





