Advances in Animal and Veterinary Sciences
Research Article
The Protective Impact of Vitamin E against Atenolol Effect on Reproductive Efficiency in Male Rats
Safa M Abbas, Luma W Khalil*
Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Iraq.
Abstract | This study was designed to assess the ameliorative role of vitamin E on sperm parameters, reducing male fertility due to oxidative stress induced by atenolol in adult male rats. Thirty-two adult male rats were divided into four equal groups (C, T1, T2 and T3). Where, group C (control group), group T1 treated orally with atenolol, group T2 given atenolol plus vitamin E orally, group T3 given only vitamin E orally. Heart blood was collected at zero and 56 days. Following parameters were studied: Total cholesterol concentration, peroxy nitrate concentration and reduced glutathione (GSH) concentration. Histopathological examination of testes and epididymis, Sperm viability, gonadosomatic index (GSI) and sperm DNA damage were done at 56 day.Results; results showed a significant elevation in serum total cholesterol and peroxy nitrate in group (T1), while these levels beginning to lowering significantly in group (T2). A significant (P<0.05) decreasing in (GSH) concentration with a significant (P<0.05) increasing to peroxy nitrate in group (T1) as compared with group (T2) which showed a significant (P<0.05) increasing in levels of (GSH). Histopathologically, atenolol affects the testes and epididymis by causing edema in the interstitial tissue with atrophy of leydig cells, incomplete spermatogenesis as comparing to group (T2) which showed complete spermatogenes process and sperm in the lumen of seminiferous tubules. Sperm DNA damage analysis of group (T1) showed marked induction of chromosomal damage as compared with vitamin E that ameliorates these harmful effects. In conclusion, vitamin E is a powerful antioxidant that ameliorates the deleterious effects on male reproductive system in patient treated with atenolol.
Keywords | Atenolol, Vitamin E, Testis, Epididymis, Male reproductive system
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | January 22, 2017; Accepted | March 04, 2017; Published | March 20, 2017
*Correspondence | Luma W Khalil, Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Iraq; Email: waleedluma@yahoo.com
Citation | Abbas SM, Luma WK (2017). The protective impact of vitamin E against atenolol effect on reproductive efficiency in male rats. Adv. Anim. Vet. Sci. 5(3): 133-138.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.3.133.138
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Abbas and Khalil . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Beta-blocker is first-line of treatment for hypertension and is regularly utilize treating hypertension since of their safety and capability in decreasing mortality and morbidity. (Turner et al., 2010) DNA fragmentation is one of the most important reasons of male infertility. Different clinical studies show that level of fragmentation of the chromatin above30% is related with spontaneous abortion or no pregnancy. (Carrell et al., 2003) Moreover antihypertensive agent atenolol was found out to induce chromosomal aberration and sexual dysfunction, impotence (Manolis et al., 2012) oxidative stress and sperm DNA damage (Rocco et al., 2012) However, the prolonged administration of atenolol causes reduction in serum glutathione (GSH) concentration because it has minimal effects on angiotensin II production in the body through the inhibition of rennin angiotensin system which acting an essentially to diminishing GSH (Haidar et al., 2014) which has influencing sperm DNA integrity to produce chromosomal abnormality and thus influence sperm parameters (Sakr et al., 2013). The stimulatory effect of catecholamine’s on lipolysis is mediated by beta receptor stimulating property. However, treatment with cardioselective β-blockers like atenolol causes a raise in plasma TGL and cholesterol levels. Vitamin E (alpha-tocopherol) is a fat soluble vitamin which regulates different oxidation processes in the body as it acts as a non-specific series-breaking antioxidant that found in cell membranes and obscures the propagation of free-radical reaction, the vitamin is a peroxyl radical scavenger and particularly ensures polyunsaturated fatty acids (PUFAs), within membrane phospholipids and in plasma lipoproteins (Shalaby et al., 2010). In addition, vitamin E possesses powerful neuroprotective, anticancer and cholesterol properties also there is a possible relation between the molecular mechanisms involved in formation and repair of DNA breaks with tocohormonal mixture supplementation. So this protective role of vitamin E well enhancing the adverse effect of atenolol on fertility impairment and improper semen parameters. Therefore, this study was designed to investigate the possible protective effect of vitamin E on reproductive efficiency in male rats exposed to atenolol.
Materials and Methods
Experimental Animals
Sexually mature male Wistar albino rats weighing 150 ±10g were used in the present study from the animal’s house of Al-Dyoania University. Animals were kept in the laboratory under constant temperature (25 ± 3ºC) throughout the experimental work. They were maintained on a standard rodent diet composed of 20% casein, 15% corn oil, 55% corn starch, 5% salt mixture and 5% vitaminized starch (green world Company, Iraq). Water was available ad libitum. Maintenance of animals and experimental procedures was approved by the animal ethical committee in accordance with the guide for care and use of laboratory animals prepared by Baghdad University, Iraq. Thirty-two healthy adult males rats at random divided into four equal groups (8/group) and treatment for 56 days (December 2015 to February 2016) as follows: First group (control): Animals in this group received tap water and served as control. Second group (group T1): Animal in this group received atenolol orally at dose. Third group (group T2): received Vit.E orally. Vitamin E is capsule, at dose (800 IU /B.W. Kontam company,china) and atenolol is tablet at dose (100mg/human/day Ajanta company,india) (Biswas et al., 2001), the dose of vitamin E and atenolol calculated according to animal weight. Rats were regularly orally administered with atenolol and vitamin E at a dose level of (2 mg/ml and 12 IU /ml respectively). Fourth group (group T3): received Vitamin E and atenolol orally.
Parameters Studied
Serum biochemical parameters: Blood sample were collected at 0, 56days of experiment via cardiac puncture technique. Serum samples were used for measuring Total cholesterol concentration (mg/dl) as described by (Allain, 1974). Reduced glutathione (GSH) concentration (μmol/L) according to (Burtis, 1999). Peroxy nitrite radical concentration (M/L) as described by (Vanuffelen et al., 1998).
Epididymal tail sperm suspension: The sperm suspension was obtained from animals for detecting gonadosomatic index (GSI) as described by (Parandin et al., 2010),Sperm Viability according to (Laing et al., 1979) and DNA Damage Analysis (Agarose Gel Electrophoresis) as described by (Couto et al., 2013) and measure its concentration.
Histological Studies: For light microscopic studies, animals were dissected and their testes were removed and fixed in 10% neutral formalin for 24h, washed in running tap water for 24h and transferred to 70% ethyl alcohol. Tissues were dehydrated in ascending series of ethyl alcohol, cleared in xylene and embedded in wax. Sections of 5 microns thickness were cut using rotary microtome and mounted on clean slides without using any adhesive medium. Sections were stained with Ehrlich’s haematoxylin and counterstained with eosin and photographed.
Statistical Analysis
Results are expressed as mean ±SE. Statistical analysis of data was performed using two-way analysis of variance (ANOVA II), Group differences were determined using least significant difference (LSD) test at P<0.05 (Snedecor et al., 1973)
Results and discussion
Serum Biochemical Parameters
Table 1 shows significant (p<0.05) increase in the mean value of in serum TC at 56 days of experiment in T1 group as compared to the control, and T2, meanwhile significant decrease (P<0.05) in serum TC at T3 group Likewise, TC concentration decreased significantly (p<0.05) in rats received atenolol concurrently with vitamin E group (T2) and vitamin E group (T3). The results reported a significant change in the levels of total cholesterol caused by potential mechanisms involving that atenolol interfere with insulin secretion from pancreatic beta cells via impairment of beta2-mediated insulin release Since lipoprotein lipase is activated by insulin therefore, impairment of insulin secretion and/or action could be the cause of dyslipidemia caused by atenolol (Sarafidis et al., 2006). Treated groups with Vitamin E showed that a significant decrease in serum total cholesterol in atenolol plus vitamin E treated group due to oral administration of vitamin E could reduce the serum level of total cholesterol. The possible mechanism for this effect is that vitamin E has the capacity for protection of membrane bound lipoprotein-lipase against lipid peroxide. Moreover, vitamin E could be increased the activity of enzyme lecithin cholesterol acyltransferase (LCAT). This enzyme may increase the transport of cholesterol ester from tissue to liver and stimulate the production and release of HDL in blood (Gumus and Hilt et al., 2016).
Table 1: Effect of Vitamin E on total cholesterol concentration (mg/dl) in admale rats treated with Atenolol. Mean ± S.E. (n =8)
|
Groups Times |
Control group |
Group T1 (Atenolo) |
Group T2 (Atenolol+VitE) |
Group T3 (Vit E) |
|
Zero day |
57.67±1.72 |
56.64±1.72 a |
58.63 ±4.10 |
58.66 ±1.71 B |
|
56 days |
58.67±1.73 B |
45.55±2.29 C b |
61.73±0.34 B |
76.62±0.56 A a |
T1: Animals received 100 mg/kg.B.W.atenolol in tap water; T2: Animals received atenolol and vitamin E; T3: Animal received 800IU/B.W. vitamin E. Capital letters denote differences between groups, (P<0.05) and Small letters denote significant differences withintime (P<0.05).
Table 2 shows significant (p<0.05) decrease in GSH concentration at day 56 of the experiment in T2 group compared to the control and T3 groups and significant (P<0.05) increase in serum GSH concentration at 56 days of the experiment in rats received vitamin E (group T3). Table 3 shows significant(p<0.05) increase in the value of serum peroxy nitrite concentration after 56 days in atenolol treated group(T1) as compared to control and T2 groups and significant (p<0.05)decrease in value of serum peroxy nitrite concentration in vitamin E treated group (T3).
Table 2: Effect of Vitamin E on serum reduced glutathione (GSH) concentration (µmol/L) in adult male rats treated with Atenolol. Mean ± S.E. (n = 8).
|
Groups Times |
Control group |
Group T1 (Atenolo) |
Group T2 (Atenolol+VitE) |
Group T3 (Vit E) |
|
Zero day |
78.56±1.27 |
77.05± 0.60 b |
75.32±0.62 |
78.65±0.60 |
|
56 days |
79.43±1.94 C |
121.78±1.58 A a |
88.57±1.03 B C |
75.01±0.89 D |
T1: Animals received 100 mg/kg.B.W.atenolol in tap water; T2: Animals received atenolol and vitamin E; T3: Animal received 800IU/B.W.vitamin E. Capital letters denote differences between groups, P<0.05 and Small letters denote significant differences within time (P<0.05).
Effect of atenolol on GSH and peroxynitrate, we explored the possibility that hyponatremia which is result from the depression of atenolol to the renine- angiotensin system may induce oxidative stress, as observed in findings which refers that hyponatremia triggered oxidative stress (Barsony et al., 2010). Concomitantly, one of the common side effect of atenolol is reduce CO-Q10, a powerfull antioxidant in the memberan of mitochondria. (Lenaz et al., 2007). So, oxidative stress increase during atenolol treatment, leading to decreasing in the level of GSH concentration and increasing in the value of serum peroxy nitrite concentration. Intubation of vitamine E to rats caused a significant increase in serum GSH concentration and a significant decrease in the serum peroxinitrite concentration, vitramine E was found to exhibit a protective effect on the testis of rats. it is a major chain-breaking antioxidant in the sperm membranes, the majority of vitamin E function is probably attributed to tocopherol to prevent the propagation of free radical reactions by acting as a peroxyl radical scavenger and protecting polyunsaturated fatty acids within membrane phospholipids and in plasma lipoproteins.So effect of vitamin E in stabilizing,exert through off-binding vitamin to membrane phospholipids on its lipophilicroperties (Monsen et al., 2000).
Table 3: Effect of Vitamin E on serum peroxy nitrite radical concentration (µmol/L) in adult male rats treated with Atenolol. Mean ± S.E. (n = 8)
|
Groups Times |
Control group |
Group T1 (Atenolo) |
Group T2 (Atenolol+VitE) |
Group T3 (Vit E) |
|
Zero day |
36.44±0.94 |
36.40± 0.92 b |
36.41±0.98 b |
36.39±0.90 a |
|
56 days |
36.43±0.93 C |
51.86 ±1.07 A a |
41.62±0.76 B a |
33.60±0.93 D b |
T1: Animals received 100 mg/kg. B.W. Atenolol in tap water; T2: Animals received atenolol and vitamin E; T3: Animal received 800IU/B.W.vitamin E. Capital letters denote differences between groups, P<0.05 and Small letters denote significant differences within time (P<0.05).
Table 4: Effect of Vitamin E on sperm viabile percent in adult male rats treated with Atenolol. Mean ± S.E. (n = 8).
|
Groups Times |
Control group |
Group T1 (Atenolo) |
Group T2 (Atenolol+VitE) |
Group T3 (Vit E) |
|
Sperm viability (%) |
81.009±0.56 B |
67.50±0.84 D |
72.50±0.85 C |
92.00 ±0.56 A |
T1: Animals received 100 mg/kg.B.W. Atenolol in tap water; T2: Animals received atenolol and vitamin E; T3: Animal received800IU/. B.W.vitamin E. Capital letters denote differences between groups, (P<0.05).
Table 5: Effect of Atenolol, Vitamin E and Atenolol plus Vitamin E on gonadosomatic index in adult male rats. Mean ± S.E. (n = 8)
|
Groups Times |
Control group |
Group T1 (Atenolo) |
Group T2 (Atenolol+VitE) |
Group T3 (Vit E) |
|
After 56 days of treatment |
0.73 ±0.09 B C |
0.72 ±0.09 C |
0.74 ±0.05 B |
0.78±0.15 A |
T1: Animals received 100 mg/kg. B.W. Atenolol in tap water; T2: Animals received atenolol and vitamin E; T3: Animal received 800IU/B.W.vitamin E. Capital letters denote differences between groups, (P<0.05).
Gonadosomatic Index (GSI) And Sperm Viability (Live Sperms Percentage)
The results of this work show that Compared to control group, atenolol treatment did not affect the gonadosomatic index (GSI) significantly, also the result gives the variation in the sperms viability in the all treated groups, a significant decrease (P<0.05) in sperm viability at 56 days of treatment with atenolol, also within the time, a significant (P<0.05) increase in sperm viability were observed in T3 treated groups comparing to control. Also the effect of oral intubation of atenolol (T1), atenolol plus vitamin E (T2) and vitamin E (T3) on gonadosomatic index are clarified a significant (P<0.05) decrease in gonadosomatic index compared to other groups throughout the experiment. Our results show atenolol treatment did not affect the gonadosomatic index (GSI) significantly and this results reinforced with (Lin et al., 2015). A significant decrease in sperm aviability was detected in group T1 after 56 days of treatment with atenolol may be due to oxidative insult which effect on sperm DNA and male fertility and could be effect on spermatogenisis and steroid biosynthesis of Leydig cells (Sakr et al., 2013) or may be due to the effect of atenolol on concentration of testosterone (Khan et al., 2004) promote spermatogenesis and maintenance of sperm production (Pineda et al., 2003). This may be attributed to the antioxidant effect of vitamin E which is responsible for its protective effect against oxidative stress, so spermatozoa are protected by antioxidant properties of vitamin E, which removed free radical and improve the sperm viability and gonadosomatic index (GSI) (Momeni et al., 2009).
Histological Observation
The typical features of normal seminiferous tubules, sper
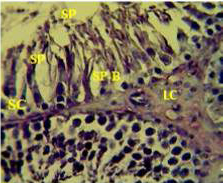
Figure 1: Section in the testis of control animal showing normal arrangement of seminiferous tubules, Leydig cells (LC) in the interstitial tissue, sertoli cells (SC), spermatogona A and spermatogona B (SP-B), spermatocytes (SP-A), spermatozoa (SP) in their lumen. (H and E stain 40X).
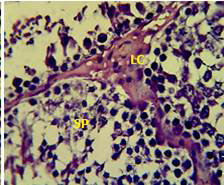
Figure 2: Section in the testis of animal treated with atenolol showing edema in the innterstitial tissue with atrophy of leydig cells (LC), incomplete spermatogenesis (SP) separated of epithelial cells from basement membrane with large spaces between few epithelial cells. (H and E stain 40X).
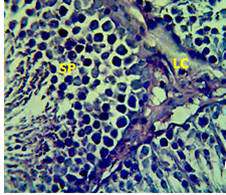
Figure 3: Section in the testis of animal treated with Atenolol and vitamin E showing edema with proliferation of leydig cells in the interstitial tissue (LC) and complete spermatogenes process and sperm in the lumen of seminiferous tubules (SP) (H andE stain 40X).
matogenic cells, intertubular connective tissue and spermatozoa were appeared in sections of testis of control rat (Figure 1) edema in the innterstitial tissue with atrophy of leydig cells,incomplete sperma togenesis separated of epithelial cells from basement membrane with large spaces between few epithelial cells shown in sections of animals given atenolol (Figure 2) also proliferation of leydig cells in the interstitial tissue and complete spermatogenes process showen in section in the testis of animal treated with atenolol and vitamin E (Figure 3) and proliferation of leydig cells with active complete spermatogenesis in section in the testis of animal treated with vitamin E (Figure 4) Various mechanisms were suggested to be responsible for histological change in testis and epidydmus. One of these mechanismsincludes increase the oxidative stress by free radical toxicity that caused by atenolol by hyponatremia and reduction in antioxidant CO-Q10 in seminal fluid and GSH. (Quinzii et al., 2007) so this testicular oxidative stress lead to a increase in germ cell apoptosis and subsequent hypospermatogenesis endocrine signaling. In addition, atenolol can reduce the the level of testosterone acutely reduced in a number of conditions associated with ROS production and oxidative stress in the testis. (Tijani et al., 2010) Vitamin E was found to exhibit a protective effect on the testis of rats,it was increased the activity of some antioxidant enzymes, decreased nitric oxide content and lipid peroxidation products in the testis protecting sperm DNA from oxidative stress of free radicals and improving fertility (Rocco et al., 2012).
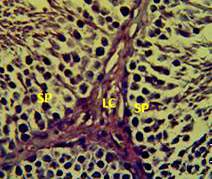
Figure 4: Section in the testis of animal treated with vitamin E showing proliferation of leydig cells (LC) with active complete spermatogenesis (SP). (H andE stain 40X).
Detection Of DNA Damage By Agarose Gel Electrophoresisand And Measure Its Concentration
The results showed that animals treated with atenolol showed a significant increase in DNA fragmentation appeared as strongly damaged Line while animals given atenolol and vitamin E showing improvement in DNA by decreasing the fragmentation and increasing the condensation and concentration as detected by Agarose Gel Electrophoresis.
The results showed that animal group which treated with atenolol are exposure to sperm DNA damage by oxidative stress in sperm DNA and for this reasons we noted the de

Figure 5: Digital printout of an agarose gel electrophoresis Showing lane (1): DNA from control sperm, lane (2) sperm DNA from atenolol treated group, lane (3) sperm DNA from atenolol plus vitamin E group, lane (4) sperm DNA from vitamin E treated group.
Table 6: Effect of Vitamin E on dsDNA Sperm Concentration (ng/ul) in admale rats treated with Atenolol. Mean ± S.E. (n =8)
|
Groups Times |
Control group |
Group T1 (Atenolo) |
Group T2 (Atenolol+VitE) |
Group T3 (Vit E) |
|
dsDNA concentration |
140.1±0.05 B |
55 ±0.05 D |
80.3 ±0.02 C |
147±0.01 A |
T1: Animals received 100 mg/kg.B.W.atenolol in tap water; T2: Animals received atenolol and vitamin E; T3: Animal received 800IU/B.W.vitamin E. Capital letters denote differences between groups, (P<0.05).
creases in a viability of sperm, several studies support this results (Roco et al., 2012) found there is Genomic Damage in human sperm cells exposed in vitro to environmental pollutants like atenolol by using different assay and test for DNA damage. Other study (Sakr et al., 2013) showed that animal given rytmonorm for five weeks showed an increase in the mean number of structural chromosomal aberration, also antihypertensive drug atenolol was found to induce chromosom loss. In addition animal group that treated with atenolol plus vitamin E shows there is less sperm DNA damage due to that vitamin E ameliorate the side effect of atenolol on sperm DNA and allows free radicals to abstract a hydrogen atom from the antioxidant molecule rather than from polyunsaturated fatty acids, thus breaking the chain of free radical reactions, the resulting antioxidant radicals being a relatively unreactive species (Aldana et al., 2001). In many studies vitamin E neutralizes lipid peroxidation and unsaturated membrane lipids because of its oxygen scavenging effect (Al-Attar et al., 2010). Therefore vitamin E supplementation is an essential component of the testis to reduced testicular oxidative stress and DNA damage.
Determination Of Sperm DNA Concentration
Table 6 showed a significant decrease (P<0.05) in dsDNA concentration at 56 days of treatment with atenolol and a significant (P<0.05) increase in dsDNA concentration were observed in T2 treated groups.
The average dsDNA concentration value obtained from the nanodrop 8000, support the data the present in gel electrophoresis. Indeed , vitamin E through its antioxidant effect ameliorate the side effect of atenolol on sperm DNA and protect it from the oxidative stress which produced from the exposure to high dose and for long period to atenolol.
In conclusion, the results suggest that administration of vitamin E to rats with oxidative stress induced by atenolol may be beneficial for reducing this side effect. The data of this study also refers that administration vitamin E alone for normal rats have protective effect on reproductive system.
Acknowledgments
Authors acknowledge to the department of physiology and pharmacology, college of veterinary medicine, Baghdad University, Baghdad, Iraq.
Conflict Of Interest
There exists no conflict of interest.
Authors Contribution
All the authors have contributed equally in terms of giving their technical knowledge to frame the article.
References





