Advances in Animal and Veterinary Sciences
Research Article
Haemato-Biochemical Profile and Milk Leukocyte Count in Subclinical and Clinical Mastitis Affected Buffaloes
Sarvesha Krishnappa1, Mayasandra Lakshmikanth Satyanarayana1, Hogalagere Doddappaiah Narayanaswamy1, Suguna Rao1, Sreenivasa Yathiraj3, Shrikrishna Isloor2, Shivalingappa Yamanappa Mukartal2, Saurabh Gupta4, Shoor Vir Singh4*, Anuradha Menon Elattuvalappil3, Kuldeep Dhama5, Srikanth Mallaiah1
1Department of Pathology; 2Department of Veterinary Microbiology; 3Veterinary College, Hebbal, Karnataka Veterinary Animal and Fisheries Sciences University (KVAFSU), Bengaluru-560024; 4Division of Animal Health, Central Institute for Research on Goats (CIRG), Makhdoom, PO-Farah, Mathura-281122, Uttar Pradesh, India; 5Division of Pathology, Indian Veterinary Research Institute, Bareilly, Uttar Pradesh, India.
Abstract | The study was conducted to determine the effect of subclinical mastitis (SCM) and clinical mastitis (CM) on haemato-biochemical parameters and milk leukocyte count of buffaloes. Milk and blood samples were collected from 20 healthy, 101 SCM and 15 CM affected buffaloes from Kolar and Chikkaballapur districts of Karnataka state, India. Haematology revealed significantly (P<0.05) higher average values of TLC in mastitis infected than healthy animals. Significantly (P< 0.05) lower average values of TEC, Hb and PCV were observed in SCM infected animals, however no significant (P>0.05) change were observed in values of CM infected than healthy animals. Biochemical estimation revealed significantly (P< 0.05) higher average values of Ca, Na and Mg in mastitis infected compared with healthy animals however, no significant (P> 0.05) change was observed in ALT levels. TP, K, P and Cl values were significantly (P<0.05) decreased and AST values were significantly increased in SCM infected compared to healthy animals. Total milk leukocytes and neutrophils were significantly (P<0.05) higher, while lymphocytes and macrophages population were significantly lower in mastitis infected animals. Changes in the haemato-biochemical parameters and milk leukocyte count are important indicators of the disturbed physiological or pathological state (mastitis) of the animals.
Keywords | Mastitis, TLC, Lymphocytes, Neutrophils, AST
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 12, 2016; Accepted | November 25, 2016; Published | December 09, 2016
*Correspondence | Shoor Vir Singh, Division of Animal Health, Central Institute for Research on Goats (CIRG), Makhdoom, PO-Farah, Mathura-281122, Uttar Pradesh, India; Email: shoorvir.singh@gmail.com
Citation | Krishnappa S, Satyanarayana ML, Narayanaswamy HD, Rao S, Yathiraj S, Isloor S, Mukartal SY, Gupta S, Singh SV, Elattuvalappil AM, Dhama K, Mallaiah S (2016). Haemato-biochemical profile and milk leukocyte count in subclinical and clinical mastitis affected buffaloes. Adv. Anim. Vet. Sci. 4(12): 642-647.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.12.642.647
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Krishnappa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
India is one of the leading country in milk production accounting for 17% of global milk production and buffaloes contributes more than half of the total milk produced in the country. Mastitis is a serious problem faced by the dairy farmers all over the world due to decreased milk production of cattle and buffaloes causing severe economic losses (Kumar et al., 2010). It is considered as the number one disease amongst all livestock diseases according to surveys in the field (Khan and Khan, 2006).
Bovine mastitis is a multifactorial disease seen in acute, gangrenous, subclinical and chronic forms of udder inflammation. Swollen, painful, discoloured udder, sudden decrease in milk production and change in milk composition are the typical signs of clinical mastitis. No visible signs are seen in the udder and milk of sub-clinically affected animals. In both subclinical and clinical forms there is an increase in somatic cell count (SCC) and decrease in milk production (Radostits et al., 2007) with SCC being inversely proportional to total milk yield (Khan and Khan, 2006).
The prevalence of mastitis increases with higher number of lactations, with reduced incidence in the first lactation. Changes in prevalence of mastitis may be regional, breed wise, therapeutic, managemental and various causative microorganisms in the environment (Sadashiv and Kaliwal, 2014). Failure of treatment in mastitis could be attributed to antimicrobial resistance, various causative organisms, pathological alterations in the mammary gland and lack of proper veterinary services (Adesola, 2012).
In mastitis, there is a break in the blood-milk barrier, along with impaired synthesis and secretory activity of udder epithelial cells, which alters the level of most components. The present study was undertaken to evaluate the haemato-biochemical and milk leukocyte count alterations in buffaloes affected with mastitis.
Materials and Methods
Collection of Samples and Tests
Blood samples were collected from jugular vein of 20 healthy, 101 SCM and 15 CM affected buffaloes from Kolar and Chikkaballapur districts of Karnataka state, India in heparinised mineral free (15-20 ml) vials and disodium EDTA (2-3 ml) vials. Blood samples were transported to the laboratory within one hour in a thermo flask with ice & then fresh blood was analyzed for Hb, PCV, TEC, TLC, ESR and DLC by using fully automated hematology analyzer (Mindray, BC-1800). The serum separated from blood was analyzed for ALT, AST, Ca, Cl, K, Mg, Na, P and TP by using fully automated biochemical analyzer (Thermo scientific KONELAB 20).
California Mastitis Test (CMT), Somatic Cell Count (SCC) and Electrical Conductivity (EC) Measurement
The milk samples collected were subjected for California mastitis test (CMT), Somatic cell count (SCC) and Electrical conductivity (EC) measurement. EC was determined by using Milk checker (Eisai Co. Ltd. and Oriental instruments Ltd., Tokyo. Japan) and a value more than 6.5 mS/cm were considered as positive for SCM (Swarup et al., 1989). The milk samples were collected aseptically in sterilized plastic bottles for SCC and differential leukocyte count. Milk total somatic cell and differential leukocyte count were estimated according to general principle of Prescott and Breed method as detailed by Schalm et al. (1971) and a value > 5.00 Lakhs/ml of milk was taken as criteria to declare the milk or animal as subclinically mastitic or infected.
Statistical Analysis
The data generated from the study were subjected to one way analysis of variance (ANOVA) test using Graph Pad Prism version 5 for windows.
Results
Haematology of animals revealed significantly (P< 0.05) higher average values of TLC in mastitis infected than healthy animals. Significantly (P< 0.05) lower average values of TEC, Hb and PCV were observed in SCM infected animals, however no significant (P>0.05) change were observed in values of CM infected than healthy animals. DLC revealed higher granulocytes and lymphopenia in mastitis infected animals (Table 1). Significantly (P< 0.05) higher average values of ESR were observed in SCM infected animals, however no significant (P>0.05) change were observed in values of CM infected than healthy animals.
Table 1: Mean ±SE values of haematological parameters of control and mastitic Buffalo
|
Parameter |
Control (n=20) |
Mastitis |
|
|
Sub clinical (n=101) |
Clinical (n=15) |
||
|
Hb g/dL |
10.45±0.30a |
8.96±0.15b |
9.66±0.45ab |
|
PCV % |
31.73±0.43a |
28.67±0.22b |
27.79±0.53b |
|
TEC x106/µL |
7.22±0.17a |
6.87±0.05b |
7.17±0.09ab |
|
TLC x103/µL |
6.82±0.23a |
10.61±0.15b |
11.32±0.47b |
|
Granulocyte % |
35.89±0.78a |
65.60±0.26b |
70.76±0.95c |
|
Lymphocyte % |
60.43±0.65a |
30.56±0.29b |
25.21±0.97c |
|
Monocyte % |
3.68±0.43 |
3.84±0.19 |
4.03±0.48 |
|
ESR mm/2hrs |
1.17±0.02a |
2.02±0.04b |
1.10±0.02a |
Means marked with different superscript a,b,c differ significantly (P< 0.05) in a row
Table 2: Mean ±SE values of biochemical parameters of control and mastitic Buffalo
|
Parameter |
Control (n=20) |
Mastitis |
|
|
Sub clinical (n=101) |
Clinical (n=15) |
||
|
ALT U/L |
9.94±0.31 |
9.99±0.14 |
9.49±0.46 |
|
AST U/L |
77.96±2.47a |
120.07±1.43b |
86.26±2.10a |
|
TP g/dL |
7.15±0.14a |
6.72±0.07b |
7.09±0.12ab |
|
Ca mg/dL |
9.59±0.20a |
13.22±0.12b |
12.98±0.30b |
|
P mg/dL |
6.86±0.19a |
4.61±0.06b |
4.97±0.19b |
|
Mg mg/dL |
2.15±0.09a |
2.45±0.05b |
2.57±0.12b |
|
Cl mEq/L |
98.46±1.46a |
88.85±0.56b |
88.21±0.77b |
|
Na mEq/L |
139.70±0.77a |
152.88±0.61b |
153.21±1.34b |
|
K mEq/L |
4.39±0.13a |
3.79±0.06b |
4.10±0.12ab |
Means marked with different superscript a, b differ significantly (P< 0.05) in a row
Biochemical estimation revealed significantly (P< 0.05) higher average values of Ca, Na and Mg in mastitis infected compared with healthy animals however, no significant (P> 0.05) change was observed in ALT levels. TP, K, P and Cl values were significantly (P< 0.05) decreased and AST values were significantly (P< 0.05) increased in SCM infected animals, however no significant (P >0.05) change were observed in TP and K values of CM infected than healthy animals (Table 2). In CM infected animals significantly (P< 0.05) lower average values of P and Cl were observed.
The total milk leukocytes and neutrophils were significantly (P< 0.05) higher, while lymphocytes and macrophages population were significantly lower in mastitis infected animals (Table 3). Significant (P< 0.05) increase in EC of milk from SCM and CM infected animals were observed in comparison to healthy animals.
Table 3: Mean ±SE values of milk EC, SCC, DLC of control and mastitic Buffalo
|
Parameter |
Control (n=20) |
Mastitis |
|
|
Sub clinical (n=101) |
Clinical (n=15) |
||
|
EC mS/cm |
4.40±0.14a |
10.07±0.20b |
17.01±0.87c |
|
SCC Lakhs/mL |
1.81±0.10a |
8.16±0.14b |
17.25±0.24c |
|
Neutrophil % |
4.97±0.22a |
43.33±0.18b |
61.77±1.11c |
|
Macrophage % |
76.34±0.16a |
27.71±0.18b |
11.74±0.33c |
|
Lymphocyte % |
15.91±0.16a |
17.90±0.17b |
16.65±0.29a |
|
Others % |
2.79±0.18a |
11.06±0.28b |
9.84±0.84b |
Means marked with different superscript a,b,c differ significantly (P< 0.05) in a row
Discussion
Hb, TEC and PCV level of SCM buffaloes showed significant (P< 0.05) decrease in comparison to healthy animals. These findings are in accordance with the findings of Zaki et al. (2008) who reported that anaemia in mastitic buffaloes were due to decrease in Hb, RBC and PCV levels. No significant (P>0.05) changes were observed in Hb and TEC level of CM infected as opposed to healthy animals. The results corroborate with the observation of Sischo et al. (1997) who reported that PCV and Hb did not exhibit any specific trend in the animals suffering from mastitis.
Significant (P< 0.05) increase in granulocytes and total leukocyte count (TLC) along with significant (P< 0.05) decrease in the lymphocyte count were observed in buffaloes affected with subclinical and clinical mastitis. Increased TLC with increase in absolute number of monocytes, eosinophils and neutrophils in mastitis were reported by Khan et al. (1997). The ESR of SCM infected animals was found to be higher than healthy animals however, no significant (P> 0.05) change was observed in CM affected animals. These findings are in agreement with Cebra et al. (1996) which could be due to chronic infection noticed in SCM.
Significant (P< 0.05) decrease in total protein (TP) level of SCM cases compared with healthy animals were observed however, non-significant (P> 0.05) decrease was observed in CM infected animals. These findings are in accordance with the findings of Mosallam et al. (2006) who reported a notable decrease in TP values of SCM infected buffaloes. Contrary to present findings Dwivedi et al. (2004) reported that significantly (P< 0.05) higher level of TPP (6.81g/dl) and globulin (3.98g/ dl) in serum samples of mastitic cows compared with 6.11g/dl and 3.25g/dl in healthy animals, respectively. This could be due to decreased levels of albumin following inflammatory response in the body of mastitic cattle (Singh, 2000).
Serum calcium level of the SCM and CM infected animals were significantly (P< 0.05) higher than the healthy animals. This could be due to the decline in the milk yield in infected animals leading to reduced excretion of Ca in the milk (Wegner and Stull, 1978). The findings of this study were in line with Singh et al. (2014) who reported significantly (P< 0.05) increased levels of plasma Ca in buffaloes suffering from mastitis. As opposed to present findings Zaki et al. (2008) reported significant decrease in the average values of Ca in serum of mastitis infected as compared to healthy buffaloes.
Average value of phosphorous of the healthy animals were significantly (P< 0.05) higher than the SCM and CM infected animals which could be attributed to its higher secretion in milk, due to injury to the udder wall resulting in increased loss in milk which is in accordance with the observation of other researchers (Mosallam et al., 2006; Siddiqe et al., 2015). As opposed to present findings Zaki et al. (2008) reported no significant change in the average values of P in serum of mastitis infected as compared to healthy buffaloes.
Serum magnesium level of the SCM and CM infected animals were significantly (P<0.05) higher than the healthy animals. The findings of this study were in line with Singh (1999) who reported significantly (P< 0.05) higher average values of Mg in acute mastitic buffaloes. Contrary to present findings Siddiqe et al. (2015) reported significant decrease in the average values of Mg in serum of mastitic cows from that of healthy cows.
Serum sodium level of the SCM and CM infected animals were significantly (P< 0.05) higher than the healthy animals.
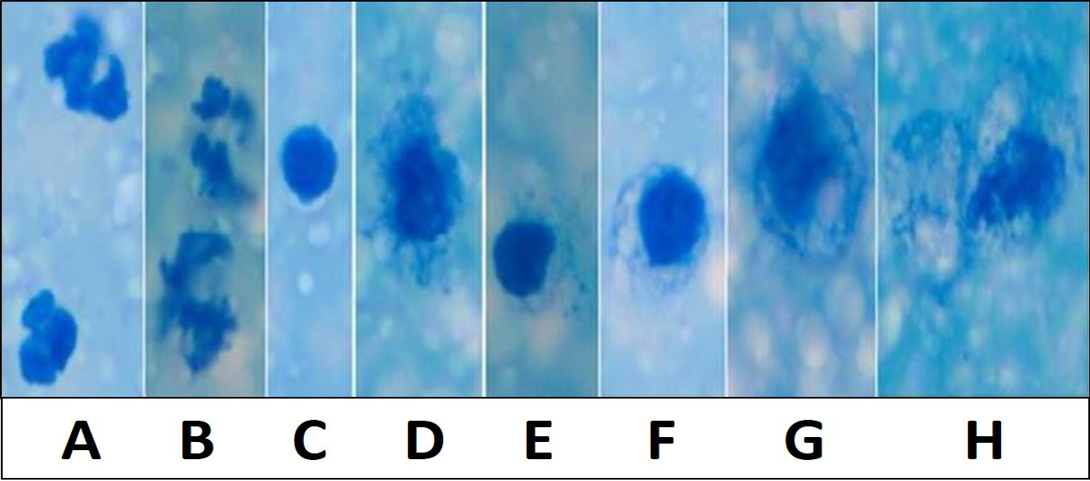
Figure 1: Various inflammatory and secretory cells Newman’s stain X 1000: A) Polymorphonuclear cells/ Neutrophils; B) Degenerating neutrophils; C) Lymphocytes; D and E) Macrophages with irregular cell membrane and basophilic engulfed material; F and G) Desquamated secretory glandular epithelial cells; H) Large desquamated secretory glandular epithelial cell with granulesMicroscopically milk showing occasional inflammatory cells in Newman’s stain X 200
The findings of this study were in line with Mosallam et al. (2006) who reported significantly (P< 0.05) higher average values of Na in mastitic buffaloes. Atroshi et al. (1996) also reported higher serum Na levels in animals suffering from mastitis. This could be due to the decline in the milk yield in animals suffering from mastitis, thereby minimizing the loss of sodium from body and elevating the level of Na in the blood.
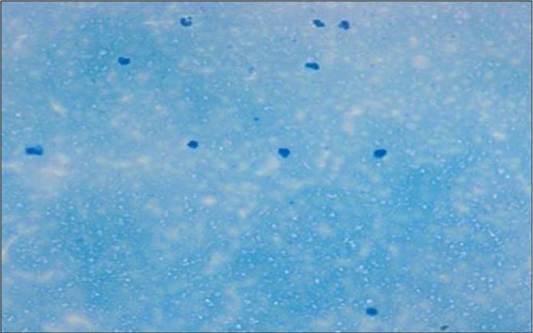
Figure 2: Microscopically mastitis infected milk showing abundant inflammatory cells in Newman’s stain X 200
Chloride and Potassium levels of the SCM animals were significantly (P< 0.05) lower than the healthy animals. These findings are in accordance with the findings of Mosallam et al. (2006). As opposed to present findings Singh (1999), reported significant increase in the levels of K in serum of mastitis infected buffaloes. This could be due to more loss of Cl and K in milk due to injury to the udder wall. The highly significant (P< 0.05) increases detected in AST values of SCM are in line with the reports of Bayumi et al. (2005) which could be due to stressful conditions.
The total milk leukocytes and granulocytes were significantly (P< 0.05) higher, while lymphocytes and macrophages population were significantly (P< 0.05) lower in mastitis infected animals indicating mammary gland infection (Figure 1 and 2). The milk leukocyte count is considered the best biomarker for inflammatory reaction in udder infection. The increased number of milk leukocyte in present study could be due to microbial infection in udder as these are the major part of the immune response of the animals (Djabri et al., 2002; Gargouri et al., 2008). These results corroborate with the observation of earlier workers (Hussain et al., 2012, 2013). The colonization of mammary glands by pathogenic micro-organisms results in a series of events which leads to major alterations of milk compositions secreted from cells. Therefore CMT (Figure 3) is a suitable measure for use on large scale monitoring programs.
Significant (P< 0.05) increase in EC of milk from SCM and CM (Figure 4) infected animals were observed compared to healthy animals. This is in accordance with the reports of Sripad et al. (2013). The increase in EC milk of mastitis animals could be due to elevated levels of ions such as sodium, potassium, calcium and chloride during mammary gland inflammation.
Conclusion
Changes in haemato-biochemical parameters and milk leukocyte count can be used as important indicators of physiological or pathological state (mastitis) of the animals. It was concluded that CMT, EC and SCC were ideal for early detection of subclinically infected quarters and aids in the selection of dairy animals for either segregation or therapy.
Acknowledgement
Authors of the manuscript thank and acknowledge ICAR NAE Project entitled: “Animal Disease Registry and Tissue Bank” Veterinary College, Hebbal, Bengaluru-24 for financial support and also authors of respective Universities/Institutes.
Conflict of Interest
Authors would hereby like to declare that there is no conflict of interests that could possibly arise.
Authors’ Contribution
All authors contributed equally.
References







