Advances in Animal and Veterinary Sciences
Research Article
Comparative Evaluation of Lateral Flow Assay and PCR for Detection of Canine Parvovirus
Kamal Hasan1, Doddamane Rathnamma1, Hogalagere Doddappaiah Narayanaswamy2 Shrikrishna Isloor1, Basavegowdanadoddi Marinaik Chandranaik4, Manayapanda Appaiah Kshama3, Anuradha Menon Elattuvalappil2, Shivalingappa Yamanappa Mukartal1, Shoorvir Singh5*, Saurabh Gupta5 and Sarvesha Krishnappa2
1Department of Veterinary Microbiology; 2Department of Pathology; 3Department of TVCC, Veterinary College, Hebbal, Karnataka Veterinary Animal and Fisheries Sciences University (KVAFSU), Bengaluru-560024; 4Institute of Animal Health and Veterinary Biologicals (IAH and VB), Hebbal, Bengaluru-560024; 5Division of Animal Health, Central Institute for Research on Goats (CIRG), Makhdoom, PO-Farah, Mathura-281122, Uttar Pradesh, India.
Abstract | Parvoviral gastroenteritis is a highly contagious viral disease which causes severe acute haemorrhagic enteritis and myocarditis in puppies over the age of 3-4 months. The present study was undertaken to detect the presence of canine parvovirus (CPV) in fecal samples of diarrhoeic dogs by conventional polymerase chain reaction (PCR) and Lateral flow assay (LFA) test followed by a comparative evaluation of the diagnostic potential of these tests. A total of 65 samples from dogs were collected including 48 faecal samples, 13 blood samples, and 4 tissue samples from different breeds and age group of animals presented to Veterinary College Hospital, Hebbal, Bengaluru. The animals were showing profuse diarrhoea with foetid odor and blood mixed faeces. Of the sixty five samples subjected to PCR and LFA, thirty and twenty samples were positive by PCR and LFA respectively. Of the thirty positive samples, seventeen were positive by both the tests and thirteen samples were positive only by PCR. The sensitivity and specificity of LFA compared to PCR was found to be 56.6% and 91.18%, respectively. The present study indicates that LFA is more sensitive than PCR and the assay can be routinely used as a rapid field level test for diagnosing CPV infections in canines.
Keywords | Canine parvovirus, PCR, Lateral flow assay
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 15, 2016; Accepted | October 15, 2016; Published | November 07, 2016
*Correspondence | Shoorvir Singh, Division of Animal Health, Central Institute for Research on Goats (CIRG), Makhdoom, PO-Farah, Mathura-281122, Uttar Pradesh, India; Email: shoorvir.singh@gmail.com, shoorvir_singh@rediffmail.com
Citation | Hasan K, Rathnamma D, Narayanaswamy HD, Isloor S, Chandranaik BM, Kshama MA, Elattuvalappil AM, Mukartal SY, Singh S, Gupta S, Krishnappa S (2016). Comparative evaluation of lateral flow assay and PCR for detection of Canine parvovirus. Adv. Anim. Vet. Sci. 4(11): 580-583.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.11.580.583
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Hasan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Parvoviral gastroenteritis is a highly contagious viral disease which causes severe acute haemorrhagic enteritis and myocarditis in puppies over the age of 3-4 months. The disease is spread from dog to dog mainly through exposure to contaminated faeces and is caused by CPV-2. It is also spread through contact with fomites (contaminated objects). It is also spread through contact with fomites. Further, large pool of unvaccinated apparently healthy stray dogs may act as carriers without showing any symptoms and become source of infection to other susceptible dogs. The stools will be watery, yellow in colour or tinged with frank blood. Faeces can vary from soft in mild cases to grossly hemorrhagic in severe cases. Death ensues due to dehydration, leading to the peripheral circulatory failure. Parvovirus myocarditis is a condition of disease is caused by Canine parvovirus-2 (CPV-2). The virus can remain on a dog’s hair coat and serve as a means of transmission long after dogs have recovered young dogs and primarily a litter problem. Within an infected litter, 70% pups will die due to heart failure by 8 weeks of age and the remaining 30% will have pathological changes which may result in death in a few months or even years later. The characteristic manifestations of myocarditis is the sudden death in young pups usually about 4 weeks of age, the collapsed dying pup may have cold extremities, pale mucosa and show terminal convulsions (Nandi et al., 2013).
Canine parvovirus is a linear single stranded DNA virus belonging to genus parvovirus of the family Parvoviridae. There are two distinct parvoviruses known to infect dogs –the pathogenic CPV-2 and CPV-1 or the canine minute virus. Canine parvovirus-2 (CPV-2) has been considered to be an important pathogen of domestic and wild canids and has spread worldwide since its emergence in 1977. Any breed of dog and wild Canines can be infected with any types of CPV, but Rottweiler, American pit bull, Small terrier, Doberman pinschers and German shepherd are more susceptible. It has been reported from Asia, Australia, New Zealand, America and Europe. In India, the first report of occurrence of CPV-2 was reported by Ramadass and Khader (1982). The virus CPV-2 is believed to be closely related to feline panleukopenia virus (FPV), mink enteritis virus (MEV) and raccoon parvovirus (RPV). These viruses exhibit over 98% homology at the level of nucleotides and amino acid sequences (Parrish and Carmichael, 1988).
Several laboratory diagnostic tests have been developed and are available for specific viral diagnosis. Rapid diagnosis can be made by electron microscopy of faecal material from cases with typical signs of diseases. Good zoo-sanitary practices in kennels, dog shelters including thorough disinfection of surfaces and personnel are important to control of CPV-2 infection. Hence, early diagnosis is focused on reliable molecular methods such as polymerase chain reaction (PCR). However, the technique needs relatively expensive equipment and reagents, which are not available in routine veterinary practice. This has led to the development of various rapid field level diagnostic test kits based on the principle of Lateral flow assay test (LFA test). The advantage is that these tests are easy to perform with minimal costs even by the dog owners (Vakili et al., 2014). Therefore, the present study was undertaken to evaluate the sensitivity and specificity of LFA in comparison with PCR as gold standard.
Materials and Methods
Collection of Samples and Tests
A total of 65 samples (48 faecal, 13 were blood, and 4 tissue) were collected from dogs of different breeds in age group of 3 months to 1 year, which were presented to Veterinary College Hospital, Hebbal, Bengaluru. Faecal samples and tissue samples were diluted in 1 ml sterile PBS and immediately shifted to the laboratory under cold chain and stored at 4°C for further processing. Blood samples were collected in EDTA containers and stored at 4°C for further processing. The collected samples were emulsified in 0.1 M PBS of pH 7.4 and centrifuged at 6000 rpm for 15 min. The supernatant was collected and used for Lateral flow assay (LFA) and PCR amplification.
Lateral Flow Assay
Lateral flow assay kit was obtained from M/s. Ubio Biotechnology systems Pvt. Ltd., Cochin. It was used to detect Canine parvovirus antigen in faecal sample as per the instructions provided on the manufacturer’s leaflet.
Polymerase Chain Reaction
All 65 clinical samples were processed for DNA isolation using the QIAGEN DNeasy Blood and Tissue kit. DNA samples were amplified using VP 2 gene specific primers H For and H Rev (Buonavoglia et al., 2001). Briefly, in a volume of 12.5 μl of 2X master mix, 1.0 μl forward primer (10 pmole/μl) and 1.0 μl reverse primer (10 pmole/μl), 5.5 μl of nuclease free water and 5 μl of template DNA was added (total volume of 25 μl). The concentration of DNA from the clinical sample to be used was titrated from 3 µl to 8 µl per reaction; concentration of 5 µl gave consistent results. Hence 5 µl of template DNA was used in the reaction which was in accordance with Paola et al. (1998). Thermal cycler conditions was as follows: initial denaturation (94°C for 10 min), followed by 35 cycles of denaturation (94°C for 30 s), annealing (55°C for 60 s), extension (72°C for 1 min) and final extension (72°C for 10 min). Amplified product (630 bp size) was analyzed by 1.5% agarose gel electrophoresis.
Statistical Analysis
Sensitivity, specificity and kappa agreement values between test combinations (LFA-PCR) were analysed by using Graph Pad Prism version 5 software USA.
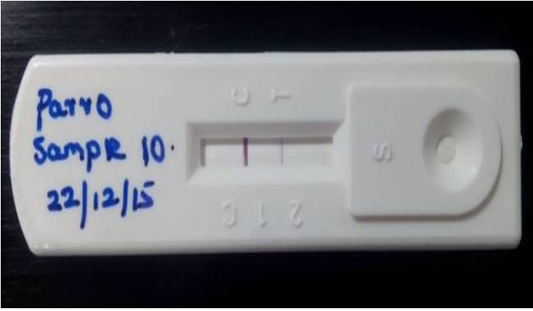
Figure 1: Detection of Canine parvoviral antigen in faecal sample by Lateral flow assay: T) Test line represents presence of CPV in test sample, C) band indicates Validity of the result
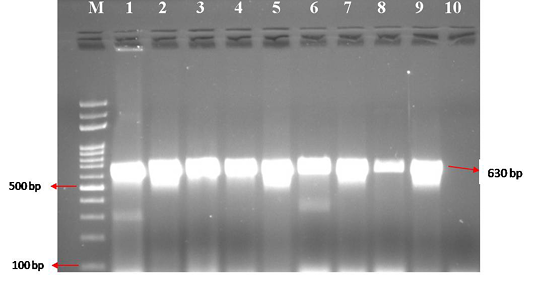
Figure 2: CPV specific amplicons (630 bp) by PCR using H specific primers: Lane M) 100 bp ladder; Lane 1) Positive control (CPV DNA); Lane 2-9) DNA samples isolated from faeces and blood; Lane 10) Negative control
Results
A total of 65 samples screened by two tests, 20 (30.7%) and 30 (46.1%) were positive by LFA and PCR, respectively (Table 1). Out of 20 samples LFA positive, 14, 3 and 3 were positive in faecal, blood and tissue respectively. Combination of tests detected 17 (26.0%), samples positive by both LFA- PCR. Value of kappa coefficient was moderate (0.493), between LFA-PCR (Table 2). On comparison, sensitivity of LFA compared to PCR was 56.6% and specificity of LFA in comparison with PCR was 91.18% (Table 1).
Table 1: Sensitivity and specificity of lateral flow assay in relation to PCR for detection of Canine parvovirus antigen
|
Test |
PCR |
Sensitivity |
Specificity |
|||
|
Positive |
Negative |
Total |
||||
|
Lateral flow assay |
Positive |
17 |
03 |
20 |
56.6% |
91.18% |
|
Negative |
13 |
32 |
45 |
|||
|
Total |
30 |
35 |
65 |
|||
Table 2: Comparative diagnostic potential of different test combinations
|
Test combinations |
Positive (%) |
Kappa value |
Strength of agreement |
|
LFA and PCR |
26% |
0.493 |
Moderate |
Discussion
Parvoviral infections in dogs has evolved as an important problem worldwide. The clinical signs resemble other enteric diseases and hence, early rapid diagnosis of the condition is critical to initiate the treatment. The most rapid method for diagnosing parvoviral infections in practice is immuno chromatography based canine faecal antigen test which is sensitive, simple and rapid (Vakili et al., 2014). In the present study, Lateral flow assay detected lower percentage of positive cases as compared to PCR. The sensitivity of LFA depends on virus load in samples. These findings were in agreement with Mallikarjun (2013) who also reported 54% positivity. Tinky et al. (2015) also reported 36% positivity for CPV infection. LFA can detect the presence of parvovirus, but will not be able to distinguish the antigenic type involved.
PCR proved to be highly specific and sensitive in detecting CPV (Schimtz and Coenen, 2009). In the present study, PCR detected more number of positive animals than LFA indicating that PCR to be more sensitive than LFA. The present findings of higher sensitivity of PCR assay in detecting CPV was in agreement with previous report of Schimtz and Coenen (2009) who stated that rapid antigenic detection test has high specificity but poor sensitivity when compared to PCR. However, when compared to the Haemagglutination inhibition assay, canine faecal antigen test kit had 97.1% sensitivity and 76.6% specificity as reported by Oh et al. (2006).
A Combination of tests can be evaluated on different set of samples to formulate the best strategy for the diagnosis of CPV infection in dogs. In the present study, LFA when used in combination with PCR was able to detect lower number of positive cases than individual test. Three samples which were positive in lateral flow assay failed to give a positive result in PCR. This could be due to the presence of inhibitory substances in faeces which might have interfered with PCR assay. This is in accordance with previous studies by Mochizuki et al. (1993) and Tinky et al. (2015) who also have reported false negative results in PCR due to the presence of inhibitory substances in faeces. The samples which were negative by LFA, were found positive by PCR assay. This could be attributed to the requirement of large amount of viral antigen to produce a clear, visible band in LFA. These findings are in agreement with that of Mohyedini et al. (2013) and Tinky et al. (2015) who have reported that quantity of viral particles in the sample can affect the lateral flow assay results which was observed to be one of the major disadvantages of this test. It is proved that samples with viral load more than 109 DNA copies per milligram faeces were generally detected by in-house assay (Decaro et al., 2010).
In the present study, sensitivity and specificity of LFA compared to PCR was found to be 56.6% and 91.1%, respectively. The results are in accordance with Vakili et al. (2014) who stated that PCR is a more sensitive test than LFA. Tinky et al. (2015) reported 72.7% sensitivity and 92.8% specificity of immune chromatography strip test tin comparison with PCR. However, Esfandiari and Klingeborn (2000) noticed that immunochromatography assay or LFA was found to be highly specific (98.8%) and sensitive (100%). Kappa statistics and calculation of agreement between LFA-PCR showed “moderate agreement” for detection of CPV infection in different number of individual samples. The current study indicated that LFA could be used as rapid field level screening test and PCR could be utilized for confirmation of individual cases.
Conclusion
Canine parvovirus is one of the most important viruses infecting dogs. One of the important factors which makes the virus a serious pathogen is its high morbidity rate. Increasing incidences of CPV-2 in vaccinated dog population is also a matter of concern with regard to prevailing CPV strains showing possible mutational escape from the vaccine strain. The study establishes the PCR to be a better test in detection of CPV-2 in clinical samples as compared to LFA and LFA test could be recommended as a rapid field level diagnostic tool for the diagnosis of CPV infections in dogs.
Acknowledgements
Authors are thankful to Indian Council of Agricultural Research (ICAR) and Karnataka Veterinary Animal and Fisheries Sciences University (KVAFSU), for provision of funding and facilities through ICAR -NAE project ‘Animal Disease Registry and Tissue Bank’, Department of Veterinary Pathology, Veterinary College, Hebbal, Bengaluru.
Conflict of Interest
We declare that we have no conflict of interest.
Authors’ Contribution
All authors contributed equally.
References





