Advances in Animal and Veterinary Sciences
Research Article
Effect of Lactobacillus acidophilus on some Physiological and Immunological Aspects in Experimentally Induced Colitis in Rats
Majida Abdul khaliq Jaafar Al qayim*, Noor Zuhair Mohammed Bakhit
Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Iraq.
Abstract | Inflammatory bowel diseases of two forms, Ulcerative colitis and Cron’s disaes cause inflammation and ulcers in the colon resulting in disruption of normal colonic function and altered physiological aspects. The present study aimed to investigate the beneficial effects of Lactobacillus acidophilus (LBA) as a probiotics in reducing the severity of this disease. Forty eight male rats were distributed into four groups, each of 12. 1st consider as control (C), 2nd and 4th (T1&T3) have colitis, 3rd and 4th (T2 &T3) administered Lactobacillus acidophilus in a dosage of (5 × 108 CFU). Colitis induced experimentally by intrarectal infusion of acetic acid. Food intake, body weight gain and food conversion ratio (FCR) recorded for 5th and 25 the days of colitis Blood samples were collected from experimental animals at the days 1, 5 & 25 of experiment. Food intake and bodyweight gain were reduced in treated animals but FCR was improved by the administration of lactobacillus acidophilus improved FCR in spite of reduction of food intake and bodyweight gain. Total protein and globulins were increased by L. Acidophilus in normal and acetic acid colitis rats. Total WBCs lymphocytes, and platelets showed a gradual increase by L. acidophilus while neutrophils increased in acetic acid induced colitis. Immunologically, Leptin increased sharply during the 1st in colitis followed by then followed by gradually decrease gradual decrease while in increased. Il-6 increased sharply in colitis and lactobasillus during 1st day Administration of L. Acidophilus results in beneficial effects on the alteration of some physiological measurements of acetic acid induced colitis and make balance between proinflammatory and anti-inflammatory response.
Keywords | Leptin, IL-6, Ulcerative colitis, Lactobacillus acidophilus, Physiological aspects
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 09, 2016; Accepted | September 09, 2016; Published | September 19, 2016
*Correspondence | Majida Abdulkhaliq Jaafar Alqayim, Assistant Professor, Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Iraq; Email: dr.majida.alqayim@gmail.com
Citation | Alqayim MAJ, Bakhit NZM (2016). Effect of Lactobacillus acidophilus on some physiological and immunological aspects in experimentally induced colitis in rats. Adv. Anim. Vet. Sci. 4(9): 476-488.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.9.476.488
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Alqayim and Bakhit. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Lactobacillus acidophilus (LBA) selected as a probiotic bacteria because criteria that: suitable and are originate from human and other mammals; can resist bile, acid, enzyme and oxygen; with high affinity to intestinal mucosa adherence ; can easily colonize potential in the gastrointestinal tract; and can produce antimicrobial substances (Lee , 2009). In addition to the immunity stimulation, including activation of phagocytosis and production of antibodies or cytokines (Galdeano and Perdigon, 2006), different strains of LBA in experiments exert a beneficial effects on the gastrointestinal homeostasis (Alqayim and Jabbar, 2015) and in healing inflammatory bowel disease (Javed et al., 2016; Nova et al., 2016). Ulcerative colitis is one of two types of inflammatory bowel diseases that cause inflammation and ulcers in the colon resulting in disruption of normal colonic function, such as maintaining of fluid and electrolytes leads to defect in absorption and motility. The specific clinical signs of ulcerative colitis is diarrhea mixed with blood, even some times it become asymptomatic. Experimentally ulcerative colitis can be successfully induced by different models among them, intrarectal administration of diluted acetic acid (Low et al., 2013).
Immunologically UC associated with abnormally responses, including increased production of pro-inflammatory cytokines particularly IL-6 and IL-8 and decreased others (Mudter and Neurath, 2007). The inflammatory reactions of Ulcerative colitis, correlated with unbalanced proinflmmatory cytokines release that effect on visceral adipose tissue activation in production of the adipokines such as leptin (Wauloga et al., 2014). During inflammatory reaction of ulcerative colitis the interaction between colon wall and adipose tissue is well studied. Adipose tissue as endocrine organs that secret different cytokines such as TNF-α and IL-6 and modulate the immune cells activity (Nishimura et al., 2009). Furthermore adipokines like leptin secreted by adipocytes promote inflammatory reaction by modulation of cytokines release from immune cells. Leptin, the hormone that secreted from adipose tissue and gut epithelial cells showed dramatically changes in human and experimental animals with ulcerative colitis, and decreased (Karmiris et al., 2006). Leptin as a cytokines, affect the secretion of TNF-α, decrease of serum leptin leads to impair of immune function, and on the contrary increased serum leptin elevates the inflammatory response (Procaccini et al., 2015).
The altered balance of these cytokines in UC is the target for development of novel compounds that either inhibit inflammatory cytokines or enhancing the counter mechanism against cytokines (Monteleone et al., 2013). Lactobacillus acidophilus as a probiotics proved immune-modulator for intestinal immunity and improve the corrosive necrosis of rats with acetic acid ulcerative colitis (Alqayim and Abbas, 2013). Species of lactobacillus bacteria exert anti-tumor effects (Zhang et al., 2016). Lactic acid bacteria species showed tumor- inhibitory and anti-proliferative effects and beneficial effects in treatment of colon cancer for their effectiveness in modulating cytokines release (Tiptiri-Kourpeti et al., 2016). The ulcerative colitis is an inflammatory reaction disease that associated with mobilization and recruitment some types of WBCs such as lymphocytes, neutrophils, and macrophages from the circulation to the colon mucosa. During ulcerative colitis there will be high level of neutrophils and lymphocytes infiltration in to the mucosal and sub mucosal area (Meek and Morton, 2016). Nentrophils are the most motile cells with short life span in the circulation it is one of the first responders of inflammatory cells. Neutrophils easily attracted by cytokines released from the damaged area and can migrate from blood stream to the tissues during acute phase of inflammation. In colitis neutrophils revealed many complex ways of inflammatory processes, in direct manner such as regulation of T-cells and dendritic cells activation as well as indirect effects on epithelial cell stability (Nemeth et al., 2016).
Lactobacillus acidophilus as it balance the intestinal microbiota and it proved by many researches as a preventive of cancer , antioxidant, improvement of nutrients absorption. Administration of L. Acidophilus to mice increased total WBCs and thrombocytes (Kang et al., 2011). Using Lactobacillus species for improving the nonspecific immunity in fish, Venkatalakshmi and Ebanasar (2015) found a significant increase in lymphocytes indicating the proliferation effects of probiotics meanwhile neutrophils were decreased because they attracted to the area where the need. Based on previous observations from researches in our laboratory, Lactobacillus acidophilus exert a positive role in healing the colon mucosa in acetic acid induced colitis, the present study was designed in accordance with these observation to highlight the proinflammatory and anti-inflammatory capability of the LBA in healing colitis via studying some physiological and immunological aspects and cytokines production that altered during the course healing of experimentally induced ulcerative colitis.
Table 1: Nutrient composition (%) of the diet (besler /turkey)
|
Nutrient Composition |
Percentage |
|
Dry matter |
88% |
|
Crude protein |
14-16% |
|
Crude fat |
2.5-3.0% |
|
Crude fiber |
18% |
|
Crude Ash |
10% |
|
Moisture |
10% |
|
Calcium |
1-3% |
|
Phosphorus |
1% |
|
Nacl |
1% |
|
Vitamin A |
5000 IU/KG |
|
Vitamin D3 |
700IU/KG |
|
Vitamin E |
30 mg/KG |
|
Metabolizable Energy |
2400 Kcal/KG |
Material and Methods
Experimental Design
The present study was conducted in the department of physiology and pharmacology in college of veterinary medicine / Baghdad university during the period from 1st Jan to 10th of Feb 2013, and designed according to the Research Ethic standards forty eight male rats with age range 10-12 weeks, were kept under a suitable condition of (21-25°C) in an air conditioned room and photoperiod of 12 hours daily. They were fed freely with standard pellet diet (Table 1). Divided randomly into (4 groups) 12 animals in each group, were placed into 3 replicate each of 4 animals and handled as follows; 1st group consider as control group (C) 2nd & 4th as T1 and T3 had colitis while 3rd group (T2) had sham colitis, At the day of starting induction of colitis T2 & T3 groups received (5 × 108 CFU) of Lactobacillus acidophilus as probiotics for 2 weeks by oral gavages needle, the experiment lasted for 25 days of colitis. Fasting blood samples collected at 1, 5 and 25 days of experiment via cardiac puncture technique from each anesthetized animal (I.M injection of Ketamine 90 mg/Kg B.W and Xylazine 40 mg/Kg B.W).
The strain of Lactobacillus acidophilus ROO52 used in the present study, a reference strain as freeze-dried powder obtained from Institute Rosella, Montreal, Canada. Obtain from Animal Sciences School, Louisiana State University Ag center (LSU) by Dr. Najim Hadi Najim.
Induction of Colitis
Acetic acid (AA) colitis was induced to rats of T1 and T3 groups by intra rectal (IR) inclusion of 1ml of 4% acetic acid (AA). Briefly after general anesthesia, a soft 8Fpediatric catheter was introduced into the anus for 6 cm and AA solution was carefully administered. Before taking the catheter out, 2ml air was applied in order to spread (AA)completely in the colon (Choudhary et al., 2001).
Assessment of Colitis
Colitis was assessed by clinical signs, macroscopic and microscopic score after 5th and 25th days of colitis induction for all the experimental animals. For each animal, the distal 10 cm portion of the colon was removed and cut longitudinally, and slightly cleaned in physiological saline to remove fecal residues, after examination of the colon feature 1cm of each sample was preserved in 10% formalin for histopathological examination (Luna and Lee, 1968).
Table 2: Colitis assessment arbitrary scale ranging by macroscopic
|
Degree |
Colitis scoring |
|
0 |
No macroscopic changes |
|
1 |
Mucosal erythema only inflammation without ulcer with wall thickening |
|
2 |
Mild mucosal oedema, slight bleeding or small erosions |
|
3 |
Moderate oedema, slight bleeding ulcers with slight inflammation of one site |
|
4 |
Two or more site of ulceration oedema and tissue necrosis |
|
5 |
Two or more site of ulceration and inflammation and ulcer extending (7 cm) along the length of colon |
Macroscopic inflammation scores were assigned based on clinical features of the colon using an arbitrary scale ranging from 0–5 as shown in Table 2 (Morris et al., 1989). For microscopic score an arbitrary scale ranging from 0–5 depending on histopathological criteria (Gue et al., 1997) as shown in Table 3. All tissue sections were examined by professional histopathologist.
Growth Indices
Body weight (gm), feed intake (gm / day), and feed conversion ratio were recorded for the durations 1-5 and 20-25days of colitis for the three replicates for each group food conversion ratio (FCR) was counted by using the consequent equation (Tacon, 1987):
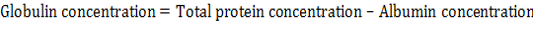
Table 3: Colitis assessment arbitrary scale ranging by microscopic
|
Degree |
Colitis scoring |
|
0 |
No microscopic histopathological appearance |
|
1 |
Loss of mucosal architecture |
|
2 |
mucosal edema, inflammatory response, cellular infiltration |
|
3 |
Submucosal muscles thickening , ulcer formation |
|
4 |
Crypt abscess formation |
|
5 |
Goblet cells depletion |
Total serum proteins, albumin and globulins: Total serum protein and albumin were measured using commercial kit product of (Biomaghreb , tunis). Serum globulin was measured by the following formula:
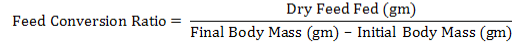
Hematological parameters: Total White Blood cells (WBCs) count, Differential counting of WBCs (%) and Total Platelets counted by auto hematology analyzer (Biobase/china).
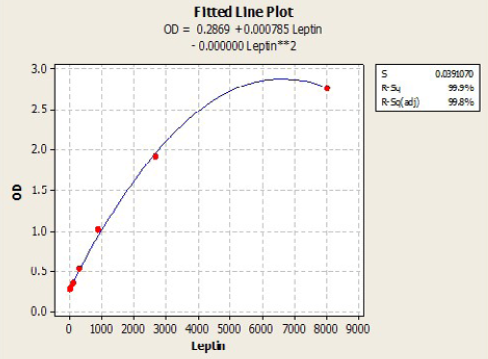
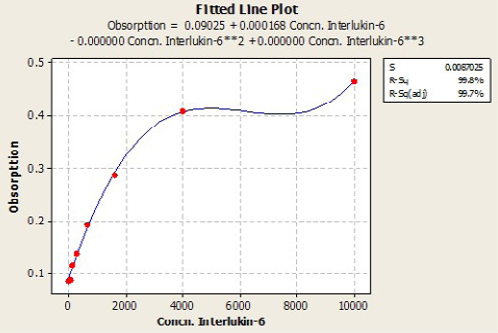
Immunological measurements: Serum IL6 (Pg/ml) and Leptin (Pg/ml) and were measured by using ELISA (RayBio / USA) Kit samples concentration calculated with standard curve plotted from absorbance of standard on the Y axis and concentration of standard on the X axis, by using plot software. According to the quadratic and cubic equation for leptin and IL-6 were drown the best fit curve (Figure 1 and 2).
Statistical Analysis
Statistical analysis achieved using SAS (SAS - Ver. 9.1)
Least significant differences (LSD) post hoc test was performed (multiple comparisons), to assess significant difference among means. P < 0.05 was considered statistically significant. The results were expressed as means ± SE.
Result
Assessments of Ulcerative Colitis by Acetic Acid
After 2 days of acetic acid colitis induction the clinical signs begins to appear, animals undergo from frequent stools, with rectal bleeding, and rectal mucous discharge, some animals marked by severe diarrhea. The macroscopic score and the gross lesion images were shown in Figure 3 and 4 shows multiple erosions of mucosa, edematous with hemorrhagic erosions scattered along the entire colon, and marked congestion of mesenteric blood vessels in T1 colon. The previous lesions were not pronounced in the proximal
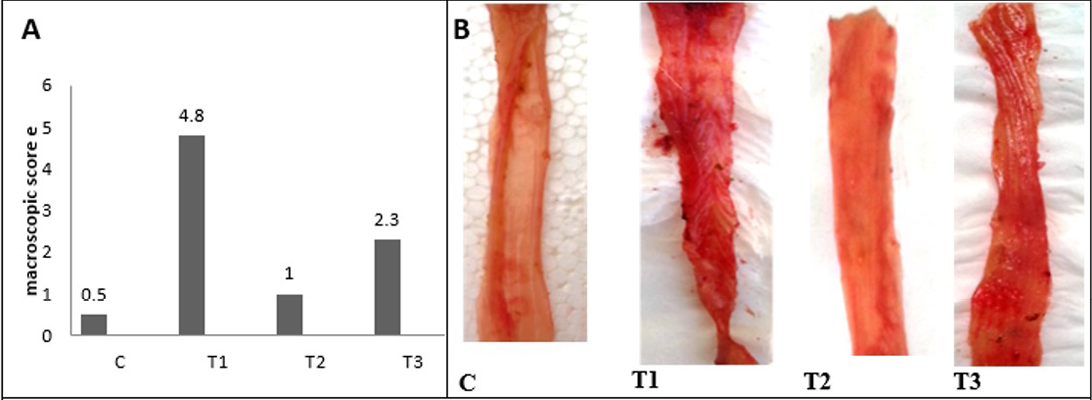
Figure 3: A) Macroscopic scoring of colitis; B) Gross lesion of colon wall after 5 days of colitis; C) control group; T1) Acetic acid colitis group, showing sever blood congestion along the colon with thickness and rough of mucosa; T2) Lactobacillus acidophilus administered; T3) Lactobacillus acidophilus administered and colitis showing small bleeding spots along the colon and thickening in mucosa and the mucosa is clearSection of intestine showing sloughing of villli and heterophillic infiltration)
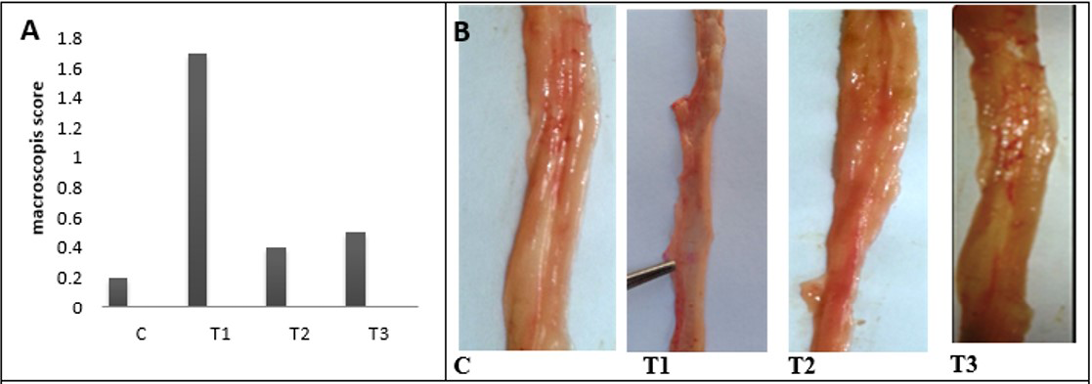
Figure 4: A) A) Macroscopic scoring of colitis; B) Gross lesion of colon wall after 25 days of colitis; C) control group; T1) Acetic acid colitis group showing thickness and rough of mucosa with large blood patches in different areas of colon; T2) Lactobacillus acidophilus administered; T3) Lactobacillus acidophilus administered and colitis showing small bleeding spots along the colon and thickening in mucosa and the mucosa is clear

Figure 5: Light microscopic photographs of colon wall sections after 5 day in C) control; T1) AA colitis; T2) LBA; T3) AA colitis +LBA; MU) mucosal ulceration; CI) cellular infiltration; AC) atrophy of crypts; LH) lumphoid tissue hyperplasia; P and L) plasma and lymphocytes, CH) crypt hyperplasia; GH) goblet cells hyperplasia; AP) Apoptosis; H and E) X100, 400
and middle colon compare with the distal colon. The colon mucosa of treated animals with LBA and had acetic acid colitis appeared less marked congestion of the colon without edema. Finally, no gross pathological changes in control and The LBA-treated animals.
The analysis of microscopic photograph of colon mucosa of present experimental animals after 5 days (Figure 5) revealed that acetic acid- colitis (T1) caused ulceration of epithelial mucosa accompany with tissue depress together with diffuse cellular infiltration in submucosa resulting in pressure atrophy of crypts and remarked goblet cells depletion, with evidence of slight lymphoid depletion, in addition to fibrino cellular exudates seen in the lumen of colon, on the contrary the predominant feature of light microscopic picture of colon wall sections of group that administered Lactobacillus acidophilus (T2) distinguish by diffuse mononuclear cell infiltration in all colon tissue, consist mainly of plasma cell and lymphocyte, accompanied by intense hyperplasia and enlargement of lymphoid tissues.

Figure 6: Microscopic score of colitis after 5 days in colon sections, (n = 24). C) control AA colitis; T1) ;T2) received (5 ×108 CFU) of Lactobacillus acidophilus; T3) received (5×108 CFU) of Lactobacillus acidophilus + AA colitis
On the other hand, the analysis of microscopic photograph of colon sections of rate have colitis and administered Lactobacillus acidophilus (T3) shows no clear pathological changes were reported, but Lymphoid tissue exhibited variable degree of lymphoid hyperplasia and appearance of few number of apoptosis, little of the crypt tissue result showed variable degree of crypt atrophy. The microscopic scoring (Figure 6) indicated that T1 and T3 had the highest score in compare with control and T2. After 25 days of colitis, results of Figure 7 shows the microscopic photograph of colon sections of the experimental animals had acetic acid colitis (T1) there was sever destruction of epithelial mucosa result in sever mucosal destruction accompany with intense goblet cell hyperplasia of crypts also multifocal lymphoid depletion observed in most section. Colon sections of T2 characterized by several blood vessels proliferation and dilation in lamina propera containing cellular infiltration together with collagen proliferation of submucosa muscularis. As well as, the thickness of sub mucosa due to presence edema with cellular infiltration and newly form blood capillary. T3 colon sections reveled moderate to severe thickness of sub mucosal tissue due to fibrinous precipitation with mononuclear cell infiltration mainly around blood vessels while the lymphoid tissue showed sever lymphoid enlargement. The microscopic scoring (Figure 8) shows that LBA administration reduced the scoring of colitis but not to normal situation.

Figure 7: Light microscopic photographs of colon wall sections after2 5 day in C) control; T1) AA colitis; T2) LBA; T3) AA colitis + LBA; MU) mucosal ulceration; CI) cellular infiltration; AC) atrophy of crypts; LD) lymphoid tissue depletion; SmT) submucosal tissue; Hand E) X100, 400
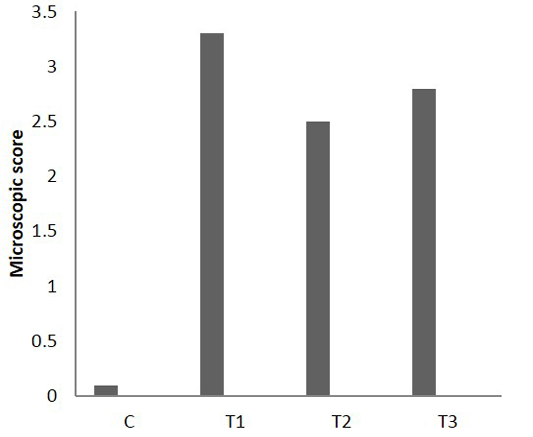
Figure 8: Microscopic score of colitis after 25 days in colon sections, (n = 24). C) Control; T1) acetic acid colitis; T2) (5 ×108 CFU) of Lactobacillus acidophilus; T3) acetic acid
Table 4: Effects of Lactobacillus acidophilus on Feed intake (g/ 5days), Body weight gain, Feed conversion ratio (FCR) in rats, mean±SE and n = 3 replicates, each of 4 animal
|
Time (days) |
control |
T1 |
T2 |
T3 |
LSD |
|
|
Food intake |
1-5 |
354.66±.59.35 Aa |
317.00±42.50Aa |
260.66±25.88Aa |
272.33±26.33Aa |
100 |
|
20- 25 |
359.66±66.68Aa |
286.00±3.05 Aa |
287.33±18.65Aa |
308.66±9.34Aa |
||
|
Bodyweight gain |
1-5 |
41.00±27.18Aa |
31.00±21.07Aa |
-19.00±3.05Bb |
-55.33±15.94Cb |
40 |
|
20- 25 |
32.33±22.98Aa |
25.33±3.17ABa |
25.33±2.18Aa |
26.00±12.12Aa |
||
|
Food conversion ratio |
1-5 |
30.36±19.28Aa |
12.6±2.38Aab |
-14.23±1.95Ab |
-6.53±3.03Ab |
33.8 |
|
20- 25 |
40.40±29.13Aa |
11.70±1.75Aab |
11.43±1.03Aab |
11.63±8.63Aab |
C: control have sham colitis; T1: AA colitis; T2: received Lactobacillus acidophilus (5 ×108 CFU); T3: received Lactobacillus acidophilus (5×108 CFU) + AA colitis; Capital letters: significant differences (P < 0.05) within group (columns); Small letter: significant differences between groups (rows)
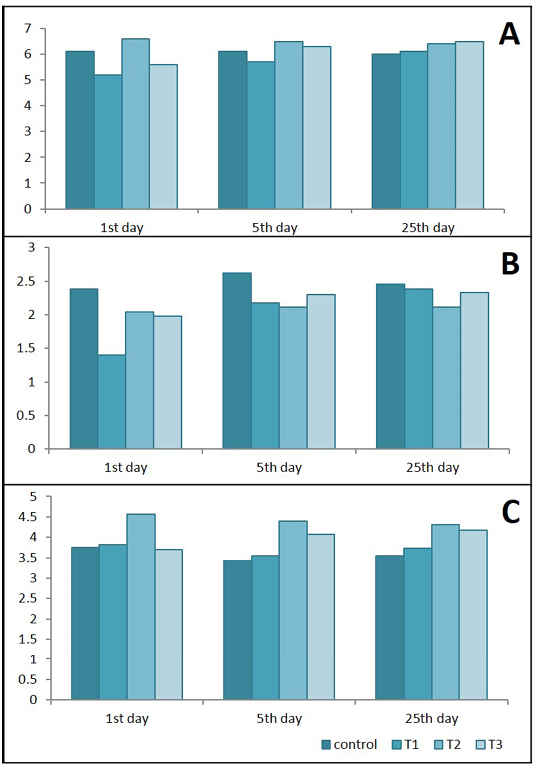
Figure 9: Effects of Lactobacillus acidophilus on A) Serum total protein(g/dl); B) Albumin (g/dl) and C) Globulins (g/dl) in rats with acetic acid colitis, mean±SE, n = 6. C) control; T1) have acetic acid colitis; T2) received (5 ×108 CFU) of Lactobacillus acidophilus; T3) have acetic acid colitis and received (5×108 CFU) of Lactobacillus acidophilus
Growth Indices
Results of the present study in Table 4, revealed a non-significant decrease in food intake in T1, T2, T3, and T2 was the lowest in food intake at 5th day of colitis, after 25 days of colitis results gave the same changes. Body weight gain decreased significantly in all experimental groups, but the most affected bodyweight gain was of T2 and T3 during the 5th days of treatment, on the other hand after 25 days of treatments the body weight gain was much better in T2and T3. The FCR decreased significantly in T1 after 5 days of colitis T2 and T3 during the 5 days of treatments, after 25 days of colitis and treatment FCR decreased significantly in T1, T2 & T3 in comprise with control.
Total Serum Proteins g/dl, Serum Albumin g/dl and Serum Globulins g/dl
Results in Figure 9 represent that total serum proteins and albumin were decreased significantly in T1 and T3 during 1st and 5th days of colitis. Serum Globulins increased significantly in T2 in different durations of experiments and at the 5th & 25th in T3.
Total and Differential White Blood Cells (WBCs)
Results in Figure 10 represents a significant elevation in total WBCs in the T1, T2 & T3 groups during the different periods of treatments when compare with control. The analysis of differential WBCs, revealed that during the 1st day of colitis caused decrease in lymphocytes with significant increase in neutrophils and monocytes while lactobacillus acidophilus succeeded in maintaining normal values in T2 and semi normal in T3. After days of treatment neutrophils and monocytes tend to decrease sharply in rats of T1 & T3 and gradually in T2 rats.
Serum Leptin and Serum IL-6
Results of the present study in Figure 11 shows that Lactobacillus acidophilus caused significant decreased in serum leptin during the 1st day of treatment. While during the 5th and 25th days of treatment this decrease was elevated to semi normal values, on the other hand colitis caused significant decrease in leptin level after 25 days of experiment. Serum level of IL-6 showed significant increase in all experimental groups (T1, T2 & T3) after day of treatments but it began to decrease in lactobacillus acidophilus administered group during the last 25 days of treatment.
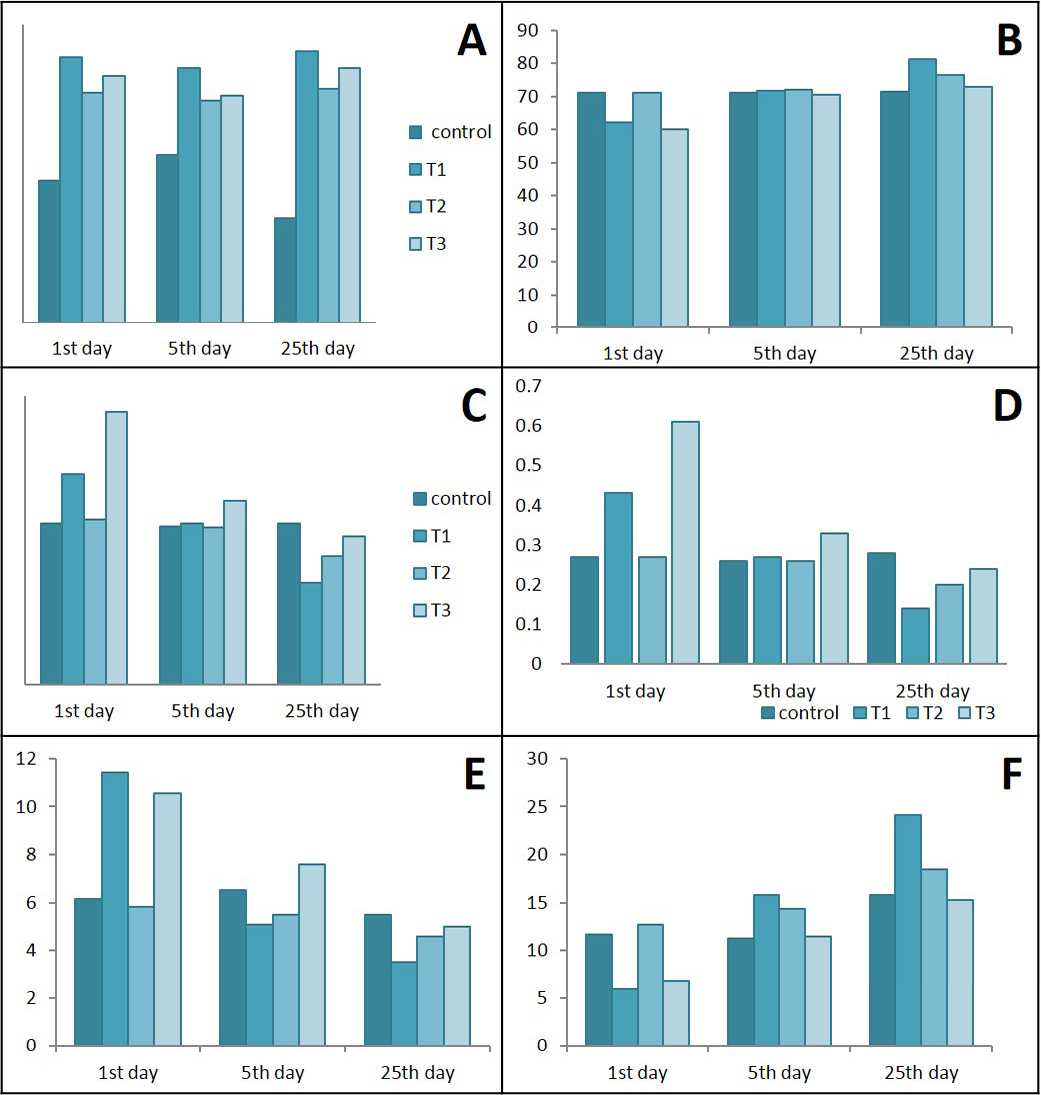
Figure 10: Ameliorative effects of Lactobacillus acidophilus on A) total WBCs; B) Lymphocytes; C) Neutrophil; D) Neutrophils / lymphocytes ratio; E) Monocytes; F) Lymphocytes / monocytes ratio in rats with acetic acid, mean±SE, n = 6. C) control; T1) have acetic acid colitis; T2) received (5 ×108 CFU) of Lactobacillus acidophilus; T3) have acetic acid colitis and received (5×108 CFU) of Lactobacillus acidophilus
Discussion
Colitis induced by intrarectal infusion of acetic acid, systemically could be consider as a stress factor that as well as locally. It’s associated effects in the colon wall as improved by the present clinical signs, macroscopic and microscopic changes of colon section in experimental animals in T1 and T3 caused a balance changes in the structure and function of the colon. These changes might be contribute to food intake and body weight dropping denoted in the present study. Abnormal integrity of intestinal wall in colitis consequently defected in the adsorptive function of the intestine leading to malabsorbance of protein and fats with anorexia (malnutrition of protein and fat) which caused decrease of protein and lipid synthesis in liver and weight loss during the first periods of experiment (Minaiyan et al., 2015).
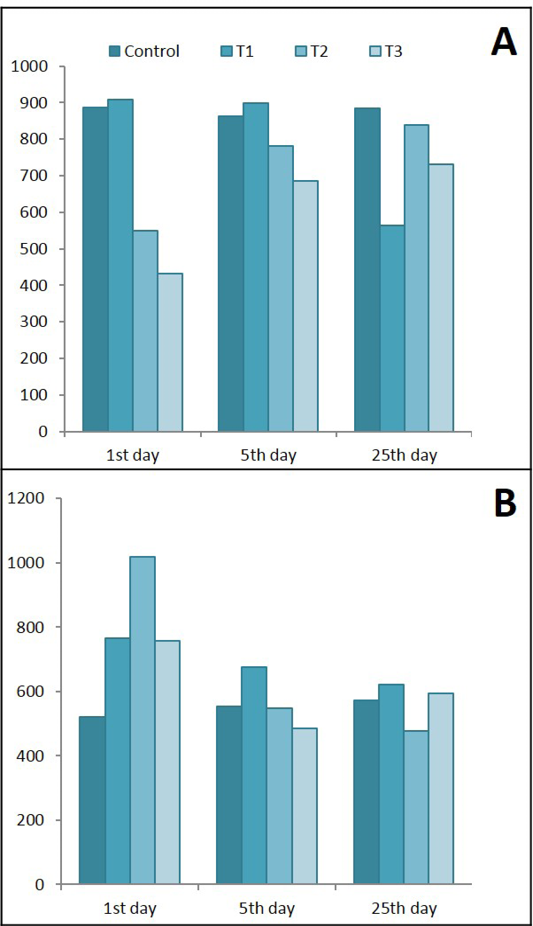
Figure 11: Ameliorative effects of Lactobacillus acidophilus on A) Serum leptin; B) Serum IL-6 in rats with acetic acid, mean±SE, n = 6. C) Control; T1) have acetic acid colitis; T2) received (5 ×108 CFU) of Lactobacillus acidophilus; T3) have acetic acid colitis and received (5×108 CFU) of Lactobacillus acidophilus
On the other hand, the effects of lactobacillus acidophilus on body weight and food intake was the subject of a difference between researchers. There is a scientific debate about the role of these bacteria as an anti-obesity, some of them consider it as anti-obesity (Sousa et al., 2008) other found that this bacteria caused increased in weight gain (Million et al., 2012). Especially when considering the fact that the actions of probiotics are always strain specific and the individual response varies according to intrinsic factors, the overall composition of diet, and their interactions (Nova et al., 2016). The previous studies revealed that administration of lactobacillus acidophilus to rats had no effect on body weight (Alqayim and Jabbar, 2015; Alqayim and Abass, 2013). Despite the reduced food intake and body weight in lactobacillus administered rats, may be indicated an improvement in the FCR. Specifically, feed conversion ratio (FCR), is a measure of an animal’s efficiency in converting the amount of feed into body gain , animals that have a lower FCR are the higher the weight gain obtained from food and are considered efficient users of feed, so lactobacillus acidophilus although it reduced the food intake but it rise the body metabolic pathway. The present results in concerned with body weight changes influenced by lacrobacillus acidophilus administration suggest a direct link between gut microbiota and brain via gut-brain axis, recent researches indicated the direct influence of probiotics with brain regions (Zhou and Foster, 2015). The intestinal microbiota has a direct effect on the brain, and the brain also influences the microbiota. This two-way gut-brain axis consists of microbiota, immune and neuroendocrine system, as well as, of the autonomic and central nervous system. Restoring normal microbiota by probiotics prevent many possible metabolic disorders like, metabolic endotoxaemia, inflammation, impaired glucose metabolism, insulin resistance, obesity and contribute to the development of metabolic syndrome, type 2 diabetes, inflammatory bowel diseases, autoimmunity and carcinogenesis (Halmos and Suba, 2016). The lowest decrease in the growth indices found in T3 could be resulted from the accumulation of influences resulting from mixed effects of colitis and lactobacillus acidophilus.
Total Serum Protein, Albumin and Globulin
In the present study the decrease of total proteins and albumin in colitis groups resulted from the malabsorption and diarrhea (Ungaro et al., 2012), could be caused by the direct effects of acetic acid on intestinal wall integrity and oxidative stress induction (Wang et al., 2016). Accordingly administration of L. acidophilus to colitis rats was efficient to improve intestinal wall integrity and consequently improve structural and absorptive features of intestine and prevent diarrhea (Minaiyan et al., 2015). These benefit effects could be attributed to either of the potential mechanisms of L. acidophilus in maintaining the flora of the gut and ongoing carbohydrate fermentation; and/or by competitively inhibiting the growth of pathogens (Oelschlaeger, 2010; Ng et al., 2009). On the other hand, because the inoculation of probiotics has been shown to increase the antibody response to gut pathogens, whereas treatment with probiotics enhance the systemic antibody response to some antigens in chickens (Hagahighi et al., 2005), and increased serum IgG concentration in broilers (Sakine et al., 2016). Furthermore, L. acidophilus enhance local production of IgA in intestinal mucosa through effects on DCs as one of the protective immunity activities of probiotics against pathogenic bacteria (Suzuki et al., 2007).
Total and Differential WBC
Because neutrophil is part of innate immunity response representing the first line of host defense therefore, it will be stimulated the first after inflammation. As part of the normal gut inflammatory response, neutrophils are recruited to sites of infection or inflammatory stimuli within minutes, and the response peaks by 24–48 hours (Soehnlein and Lindbom, 2010), resulting in reduced neutrophils percentage in the serum . Blood neutrophil-to-lymphocyte (N/L) ratio is an indicator of the overall inflammatory status of the body, and an alteration in N/L ratio may be found in ulcerative colitis (UC) that N/L ratio is higher in patients with active UC compared with controls (Celikbilek et al., 2013). In patients with UC, the blood N/L ratio is associated with active disease. N/L ratio may be used as an activity parameter in UC (Posul et al., 2015). The elevated N/ L ratio in the present study in colitis rats indicated an active disease within the first 5 days of colitis induction. While, Lactobacillus acidophilus administration tried to keep this ration in semi normal level. Lactobacillus strains caused immune stimulation by increasing the differentiating of monocytes into macrophages (Tulini et al., 2015), this immune stimulation was not inflammatory since there were low production of cytokines, Il-6 was observed after days of colitis. Monocytosis and a low L/M ratio denoted in the present study in rats suffering from colitis indicated the disease activity since the high blood monocytes and low L/M ration might be effective, readily available, and low-cost biomarkers to identify disease activity in UC patients (Cherfane et al., 2015). On the other hand, the present results shows normal to low blood monocytes and L/M ratio founded in rats administered lactobacillus acidophilus and had colitis after days of administration indicated the immunomodulator effects and reduced the inflammatory response., which may indicate role of Lactobacillus as immunobiotic they improve immunity by increasing blood monocytes (Tsukida et al., 2016).
Serum Leptin and IL-6
Serum leptin level reflect the involvement of adipokines in the pathogenesis of ulcerative colitis where they synthesized by the activated white adipose tissue or immune cells or both of them (Procaccini et al., 2015). The obliviously serum leptin increase during the first day of colitis in the present study, could be explained by the initiation of adaptive immunity mediated by lymphocytes after they had been activated by the denditic cells or the antigen presenting cells and because of pro-inflammatory cytokines (Ahima and Osei, 2004). It is believed to contribute to disease genesis and/or progression because of significant increase in leptin levels in experimental colitis that correlates with an increase in disease severity (Singh et al., 2013). Another possible mechanisms for leptin elevation there were an inflammatory stimuli acutely induce leptin mRNA by mesenteric adipose tissue and increase serum leptin levels of IBD (Barbier et al., 2003) and in experimental animals (Fantuzzi and Faggioni, 2000). During fasting period (anorexia) in colitis and after reduction of body fat mass, there is a decrease in leptin levels that leads to a reduction in total energy expenditure to provide enough energy for the function of vital organs, that is, the brain, the heart, and the liver (Fernandez-Riejos et al., 2010). Furthermore, there are strong evidences for the correlation between insulin levels as a regulator for leptin release from submucosal fat deposition. Due to mucosal inflammation by acetic acid there will be malabsorption of nutrients as glucose leading to reduced serum glucose and insulin level in the serum. The gradual fall in leptin with fasting likely depends on the drop in insulin (Fried et al., 2000). Furthermore, this sharp increase of serum leptin during the first days of induced colitis had been documented in many experimental colitis models (Deboer and Li, 2011) and children (Aurangzeb et al., 2011). Lactobacillus acidophilus acts as immunomodulator via regulation of leptin production from activated macrophages after days of administration, these results are in compatible with results of (Ivanovic et al., 2016) who reported that oral administration of active lactobacillus increased leptin and consider it as immunomodulator positive effects.
IL-6 is a typical proinflammatory cytokine whose production is induced during the acute-phase response; it is secreted by a large number of cells, and its main sources in the gut are the macrophages (Neurath, 2014). One function of inducible IL-6 during acute responses is to suppress the level of proinflammatory cytokines without compromising the level of anti-inflammatory cytokines (Kaplanski et al., 2003). IL-6 therefore can have a protective effect and modulator for the immune response (Korolkova et al., 2015). The result of the present study indicated an increase in IL-6 in the acute phase of ulcerative colitis induced by acetic acid intra rectal infusion, this increase could be attributed to the consequence of mucosal T-cell or macrophage activation (Jones et al., 1994) or IL-6 was induced early after injury in a population of intraepithelial lymphocytes (IELs) induced by acetic acid (Kuhn et al., 2014). Actually, serum IL-6 and IL-8 concentrations are often considered to be reliable markers for CD or UC and correlate with inflammatory activity (Rodriguez et al., 2012) The significant increase in serum IL-6 during the first day of treatment with lactobacillus indicated the great macrophages activation , in accordance with Galdeano and Perdigon (2006) who found when lactobacillus improved the production of IL-6 and antimicrobial activity against S. typhimurium, since activated macrophages produce large amounts of inflammatory cytokines and thus establish a pro-inflammatory environment for other immune cells in the gut (Sanchez-Muñoz et al., 2008). The present data suggest that lactobacillus acidophilus modulate the immune response not only stimulate macrophages but also modulate cytokines production , the exact mechanism for these effects need more clarifications even if recently administration of lactobacillus promote a major expression of TLRs but also use these receptors via the innate immune cells as macrophages (Galdeano et al., 2015).
One possible role of leptin in elevation of IL-6 production in acute colitis denoted in colitis group in the present study is resulted from the suggested stimulating effects of leptin on macrophages to produce more IL-6. With continued inflammation of colon mucosa without treatment the continued high level of serum IL-6 indicated its role in a variety of pathophysiology of colitis. Furthermore, we suggest here that the damaged tissue in colon mucosa caused by acetic acid resulting in disruption of tight junction and increased the mucosa permeability for the luminal AG uptake by immune cells specifically the DCs and initiation of the adaptive immunity via production of the proinflammatory cytokines release (IL-6) in high level (Ordas et al., 2012). The more damaged mucosa the more immune response the more IL-6 production.
On the other hand, the highly increase in serum Il-6 during the first day the administration of lactobacillus acidophilus of healthy and colitis rats indicated the proinflammatory activity of the LBA. Activation of the innate immunity and IgA production are thought to be the most important mechanisms involved in gut mucosa immune stimulation by probiotics (Galdeano et al., 2007).
Conclusion
Lactobacillus acidophilus showed a potential role in regulation of the inflammatory response of the colon mucosa immune cells and cytokines production, this obviously through the decrease of IL-6 in serum of rats administered LBA. This decreased IL-6 could be confirmed by the present results in regard to the micro assessment of colitis, as well as histopathological changes revealed less macrophages accumulation and immune cell infiltration in colon wall of treated rats, consequently reduced the inflammatory reactions and production of IL-6 (Novotny et al., 2015).
ACKNOWLEDGMENT
The authors would like to express their deep gratitude and appreciations to Dr. Anaam Badder for her help in the analysis and description of the histopathological part of this research.
Conflict of interest
There is no conflict of interest.
Authors’ contribution
This was the study was conducted by Noor Zuhair Mohammed Bakhit and is a part of his the MSc thesis. Majida Abdul Khaliq Jaafar Al Qayim was his supervisor and provided all the necessary support to complete the work.
References