Advances in Animal and Veterinary Sciences
Research Article
Changes in Expression of Apoptosis Related Genes and DNA Fragmentation as Biomarkers in Post-mortem Investigation of Electrocution
Mayada Ragab Farag1*, Kuldeep Dhama2
1Forensic Medicine and Toxicology Department, Veterinary Medicine Faculty, Zagazig University, Zagazig 44511, Egypt. 2Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, 243122, Uttar Pradesh, India.
Abstract | In this study, the impact of electrocution on apoptosis and DNA damage was investigated to act as possible biomarker for electric shock mediated deaths. A total of 40 adult male rats were divided into two equal groups - electrocuted (E) and Control (C), which were further sub-divided into two sub-groups. The main treatment group (E) was electrocuted until death by a 220 V alternating current (AC) and the blood and heart samples were collected immediately after electrocution from the first sub-group [E(0h)] and 1 h postmortem (PM) from second sub-group [E(1h)]. The animals of control group were killed humanely by cervical dislocation, and the samples were collected immediately after death from the first subgroup [C(0h)] and 1h after PM from the second subgroup [C(1h)]. Creatine phosphokinase (CPK) and lactate dehydrogenase (LDH) activities and malonaldehyde (MDA) contents were significantly found to be elevated in rats of electrocuted group [E(0h)] and their levels showed higher elevations in [E(1h)] group. Exposure of rats to electric current till death resulted in a highly significant increase in DNA fragmentation percent immediately or 1h of PM. The expressions of Bcl-2, Bcl-xl were down-regulated while Bax expression was up-regulated in hearts of electrocuted rats in comparison with control rats, influenced by cause of death and time of sampling. The heart taken from C(0h) and C(1h) groups showed normal architecture while more extensive damage appeared in E(0h) and E(1h) groups. In conclusion, electrocution caused DNA damage and apoptosis in cardiac muscles and these findings could be used as useful biomarkers to support biochemical analysis and histopathological examination in confirming the electrocution as the principle cause of death.
Keywords | electrocution, apoptosis, Bcl-2 family, DNA fragmentation
Editor | Yashpal S. Malik, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 01, 2016; Accepted | May 18, 2016; Published | May 30, 2016
*Correspondence | Mayada R Farag, Forensic Medicine and Toxicology Department, Veterinary Medicine Faculty, Zagazig University, Zagazig 44511, Egypt; Email: dr.mayadarf@gmail.com
Citation | Farag MR, Dhama K (2016). Changes in expression of Apoptosis related genes and DNA fragmentation as biomarkers in post-mortem investigation of electrocution. Adv. Anim. Vet. Sci. 4(5): 258-265.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.5.258.265
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Farag and Dhama. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Determining the cause of death is an important aim in forensic medicine especially when the external signs or gross findings are absent. Electrocution is one of the main causes of acute death in both human and animals (Hussmann et al., 1995). The danger of electricity returns to its wide spread and great use in our daily life. Moreover, electric current is invisible and could induce injuries at any part of the body or lead directly to death mostly without leaving any detectable marks (Arnoldo et al., 2006; Wang et al., 2008; Kurtulus et al., 2009). The degree of damage caused by electricity depends on type and intensity of electric current and the nature of exposed cells or tissues (Stone et al., 2014). The electrical shock could affect skin, central nervous system, cardiovascular system, skeletal muscles and many other internal organs (Fish, 1993a). Michiue et al. (2009) defined the ventricular fibrillation and cardiac failure as the principle cause of death in electric shock.
The most severe cardiovascular problems due to electric shock usually appear at the onset of the injury, however, some serious changes are observed in immediate post injury period (Purdue and Hunt, 1986; Fish, 1993b). In addition to the complications occurring in different organs; destruction of cell membrane and loss of resting membrane potential are important features of electric shock due to cell depolarization. Peroxidation of cell membrane has been also reported following the myocardial injury due to the induction of reactive oxygen species as a result of inflammatory reactions during the survival period (Dröge, 2002; Suematsu et al., 2003).
Some biochemical parameters could serve as confirmatory markers of myocardial damage in cases of sudden or unexplained death even in absence of histopathological findings, among them creatine phospho kinase (CPK) and lactate dehydrogenase (LDH) enzymes are important ones (Xenopoulos et al., 1991; Zack et al., 1997; Chidambaranathan et al., 2015). In some other studies, apoptosis has been also reported in early stages just with the occurrence of myocardial injury (Piro et al., 2000). Apoptosis is known to be regulated by Bcl-2 / Bax family of genes which act as key elements in the process of cell death (Nagata, 1997; Begriche et al., 2006; Berthelet and Dubrez, 2013; Kumar et al., 2015). Bcl-2 has been reported to inhibit cell death induced by different kinds of stimuli like chemotherapy and gamma radiation (Miyashita and Reed, 1993), however, its role in protecting cells from electric current still needs more investigations.
The aim of the present work focused to analyse the efficacy of some biochemical parameters, expression of some apoptosis related genes and histological changes in the heart of rat which could be used as useful biomarkers in postmortem diagnosis and confirming the electrocution as the possible cause of death in addition to investigating the DNA damaging effects of electric shock in early postmortem period.
MATERIALS AND METHODS
Animals and Grouping
A total of 40 healthy adult male Sprague-Dawely rats weighing 200±10 g were obtained from the Animal Research Unit of the Faculty of Veterinary Medicine, Zagazig University. The animals were maintained under temperature 22oC, a 12 h light/dark cycle and ad libitum availability of pellet food and water. The animals were divided randomly into 2 main groups: the control group (C) included 20 rats, divided into 2 subgroups (10 rats each) that were killed by cervical dislocation without any application of electrical current; the first subgroup was used for collection of blood and heart samples immediately after death [C(0h)], while these samples were collected one hour after postmortem (PM) in the second sub-group [C(1h)]. The electrocuted (E) group included 20 rats (in 2 sub-groups of 10 rats /each) that were electrocuted until death by a 220 V alternating current (AC) with the points of electrical contact placed on the skin of the left forelimb and the skin of the right hind limb to allow the current to pass via chest according to Wright and Davis (1980). The samples were collected immediately after electrocution from the first subgroup [E(0h)] while collected one hour after postmortem in second subgroup [E(1h)].
Tissue Preparation
The collected heart samples were divided into two parts, the first part was stored at -80˚C until analysis and the second part was fixed in 10% neutral buffered formalin for histopathological examination. Blood samples were collected from the heart and great blood vessels and centrifuged immediately for 10 min at 3000 rpm for collection of serum. Serum was stored at -20˚C until analysis.
Biochemical Analysis
Creatine Phosphokinase (CPK) Activity: CPK activity was measured using commercial diagnostic kits provided from Biodiagnostic Co. (Giza, Egypt) according to Szasz et al. (1976).
Lactate Dehydrogenase (LDH) Activity: LDH activity was evaluated using a readymade reagent kit (Bio Med Diagnostic) according to the method described by Gay et al. (1968).
Lipid Peroxides (Malondialdehyde, MDA) Content: Lipid peroxidation was determined by measuring MDA level in heart homogenate by colorimetric assay according to Ohkawa et al. (1979).
DNA Fragmentation: The DNA fragmentation assay was carried out on heart tissues of rats of all groups using cellular DNA fragmentation Elisa kit provided by Roche Diagnostics, Switzerland according to the manufacturer´ instruction.
Expression Levels of Apoptotsis-Related Genes: Bcl-2, Bcl-Xl and Bax mRNA
RNA Extraction: Total RNA was extracted from heart tissue using RNeasy Mini Kit (Cat. No. 74104 provided by (Qiagen, Heidelberg, Germany). Quantification and quality testing of the obtained RNA was done using NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, Delaware USA). Only high purity samples (OD 260/280 >1.8) were further used.
Reverse Transcription Reactions: One µg of total RNA was reverse transcribed into cDNA using Qiagen 2Step RT-PCR Kit (Cat. No. 205920) following the manufacturer instructions in a 20 µl total volume.
Table 1: Primers used for RT-PCR method for Bcl-2, Bcl-xL, Bax, and GAPDH
|
Genes |
Primers |
Product Size |
|
Bcl-2 |
F: 5´-CTG GTG GAC AAC ATC GCT CTG-3´ R: 5´-GGT CTG CTG ACC TCA CTT GTG-3´ |
228 pb |
|
Bcl-xL |
F: 5´-AGG CTG GCGATG AGT TTG AA-3´ R: 5´-TGA AAC GCT CCT GGC CTT TC-3´ |
357 pb |
|
Bax |
F: 5´-TTCATC CAGGAT CGA GCA GA-3´ R: 5´-GCA AAG TAG AAG GCA ACG-3´ |
263 pb |
|
GAPDH |
F: 5´-GGCCAAGAT CAT CCA TGA CAA CT-3´ R: 5´-ACC AGG ACA TGA GCT TGA CAA AGT-3´ |
462 pb |
PCR Amplification
The PCR mixture contained 2 μl cDNA, 0.2 mM of each dNTP, and the Taq polymerase buffer which contained 10 mM Tris-HCl pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 7.5 pM of each primer and 1.5 U of Taq polymerase, and was placed in a 2720 thermocycler (Applied Biosystems, USA). Expression was normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression, which was used as an internal housekeeping control. The list of primers sequence used was derived from Anarkooli et al. (2008) and is presented in Table 1. PCR amplification conditions were as follows: denaturation at 95°C for 4 min followed by 28 cycles of 95°C, 1 min; 55°C, 1 min; 72°C, 1 min for Bcl-2 and Bcl-xl and annealing temperature of 58°C for Bax and GAPDH. The 10 μl of PCR products were analyzed on a 2% agarose gel stained with ethidium bromide in 1X Tris acetate EDTA buffer (TAE), pH 8.3-8.5 (Stock solution was 50X from Bioshop® Canada Inc. Burlington). A 1X solution contained Tris 0.04 M, acetate 0.04M, EDTA, 0.001M. The electrophoretic picture was visualized by gel documentation system (Bio Doc Analyze, Biometra, Germany). The expression levels of the gene bands intensity on gel were analyzed using Image J software (version 1.24).
Histopathological Investigations
Heart specimens were taken from different groups and fixed in 10% buffered neutral formalin solution. Five-micron thick paraffin sections were prepared, stained by hematoxylin and eosin for histopathological examination (Bancroft and Gamble, 2008).
Statistical Analysis
Data of the current study expressed as mean ± standard error was statistically analyzed using the computer program SPSS/PC (2001). The statistical method was one way ANOVA test.
RESULTS
Effect of Electrocution on Enzymatic Activity and Lipid Peroxidation
CPK, LDH and MDA levels as affected by electrocution are summarized in Table 2. There was no significant differences between CPK level in rats of C (0h) or C (1h) groups while its level was significantly elevated in rats o electrocuted group and more elevation observed after one hour from death. LDH activity was highly significantly increased in rats of electrocuted group compared to control groups with no effect of the time of sampling. MDA content in heart of different groups was affected by the cause of death and time of sampling where the heart taken from electrocuted group at 1h postmortem showed the highest MDA level followed by heart taken immediately after electrocution while its level was lower in rats died by cervical dislocation and autopsy was done 1h postmortem, the lowest level was recorded in the samples taken immediately after cervical dislocation.
Table 2: Effect of electrocution on creatine phosphokinase, lactate dehydrogenase and malonaldehyde
|
Groups |
Biomarkers |
||
|
CPK (U/L) |
LDH (U/L) |
MDA (nmol/gm tissue) |
|
|
C(0h) |
1112.28±70.08c |
1023.85±273.4b |
60.25±1.22d |
|
C(1h) |
1194.14±174.2c |
1089.0±211.7b |
80.12±1.14c |
|
E(0h) |
2856.85±329.6b |
2907.28 ±79.3a |
119.13±1.29b |
|
E(1h) |
3377.00±365.3a |
3033.57±226.2a |
150.63±2.55a |
Effect of Electrocution on DNA Fragmentation Percent
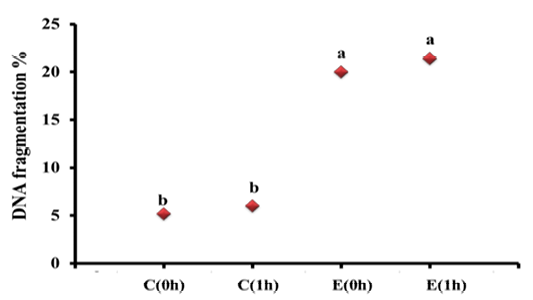
The changes in DNA fragmentation percentage among different groups are depicted in Figure 1. The DNA fragmentation percent in heart samples taken immediately after death (C0h; 5.2%) from rats killed by cervical dislocation did significantly differ from those of samples taken 1h postmortem (C1h, 6.0%). Meanwhile, exposure of rats to electric current till death resulted in a highly significant increase in DNA Fragmentation percent than control to be (20.12%) and numerically increased in samples taken from electrocuted rats at 1h post mortem to be (21.40%), indicating the extensive damage caused after electrocution.
Effect of Electrocution on mRNA Expression Levels of Apoptosis-Related Genes
The mRNA expression levels of Bcl-2, Bcl-xl and Bax inh experimental groups are illustrated in Figure 2. The expressions of Bcl-2, Bcl-xl were down-regulated in hearts of electrocuted rats in comparison with control group. Bcl-2 was affected by both type of death and time of sampling where it showed a highly significant decrease in the E1h group than all groups, while Bcl-xl was affected by only cause of death but not by time of sampling where it was significantly down-regulated in electrocuted rat hearts (E0h and E1h) than control (C0h and C1h). On the other hand, the only significant up-regulation in Bax expression was observed in E1h group compared to all other groups in which there were no changes in the expression of Bax.
Effect of Electrocution on Histopathological changes of Cardiac Tissue
Microscopical examination results of heart tissue from different groups are shown in Figure 3. The heart taken from
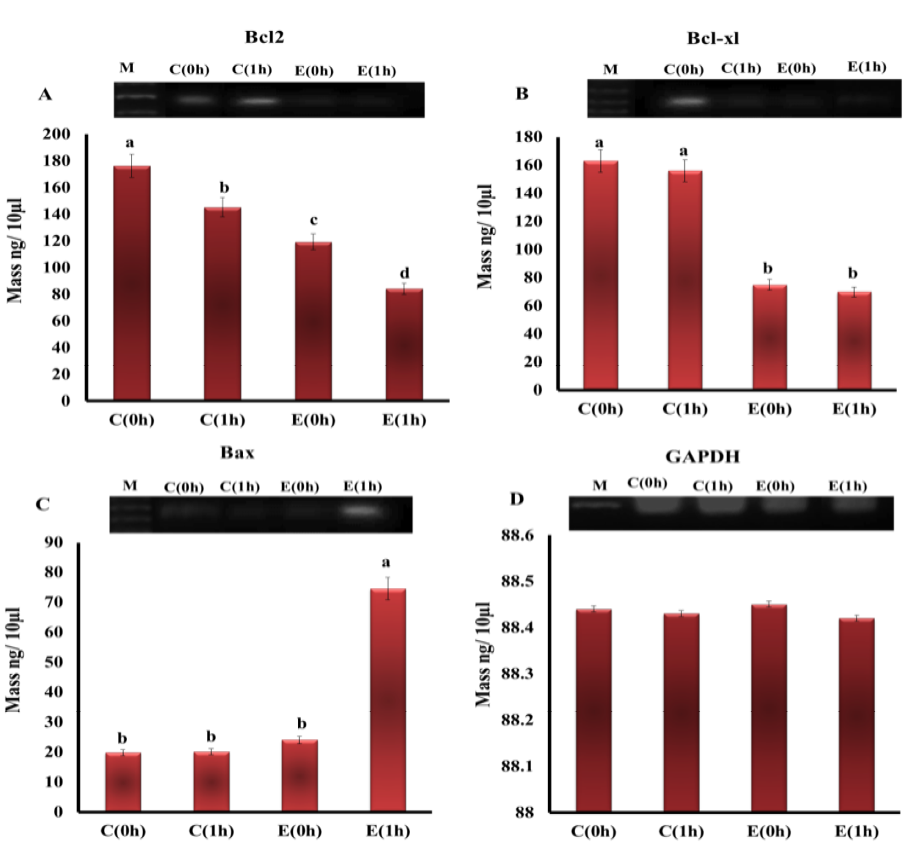
Figure 2: The expression level of mRNAs of A) Bcl-2; B) Bcl-xl; C) Bax; D) GAPDH genes in the heart tissue of rats from different groups. (M - DNA marker (100 pb))

Figure 3: Heart of control group (C0h and C1h) showed normal muscle fiber run in different directions with acidophilic sarcoplasm and oval nuclei (A and B). Heart from electrocuted group (E0h) showed necrosis and intramyocardial hemorrhage (C and D). Heart of the electrocuted group (E1h) showed necrosis, fragmentation and contraction bands (E and F)
control group immediately after death by cervical dislocation showed cardiac muscle fibers running in different directions with acidophilic sarcoplasm and central pale oval nuclei and the same findings were observed in heart tissue that taken after 1h (Figure 3A and 3B). Histopathological examination of the heart sections, taken immediately from rats exposed to electric current till death (E0h), showed areas of intermyocardial hemorrhage with necrosis in some areas (Figure 3C and 3D). While the heart taken from electrocuted group after 1h of death showed necrosis and fragmentation of some cardiac myocytes, contraction bands and myocardial waviness (Figure 3E and 3F).
Discussion
The effect of electric shock on the extent of myocardial cellular injury in the present work was investigated by measuring the levels of LDH and CPK. Exposure of rats to electric current till death resulted in a highly significant elevation in the level of LDH enzyme immediately after death and more elevation was observed in samples taken 1 h postmortem in comparison with control groups. LDH level could reflect the degree of cell necrosis and its elevated level in tissue and serum provides an index of death of cells and disturbance of their membrane permeability and indicates cell membrane disintegration and enzyme leakage (Amin and Hamza, 2005). When CPK and LDH levels are observed to be increased together, then this is considered a strong indication of myocardial damage. Similar results concerning the increased levels of LDH and CPK following exposure to electric current were obtained by Xenopoulos et al. (1991), Lichtenberg et al. (1993) and Zack et al. (1997).
MDA, the marker of lipid peroxidation (LPO), can alter the cell membrane functions and decrease cell survival by interfering with membrane phospholipids and proteins leading to cell injury and death (Sugihara et al., 1991). In the present study, MDA showed a high significant increase in response to electric shock and the peroxidative damage was higher in the group that left for an hour before autopsy than control group indicating a considerable state of oxidative damage. Lipid peroxidation that occurred in myocardial tissue may be implicated in the increased LDH in to systemic circulation. Production of reactive oxygen species (ROS) and increasing of lipid peroxides following myocardial damage has been observed earlier (Domenech et al., 2004).
Apoptosis is a type of programmed cell death which characterized by changes in cell morphology either by chromatin condensation, cell and nucleus shrinkage, blebbing of membrane or DNA fragmentation (Wyllie, 1997; Kumar et al., 2015). Apoptosis could be induced naturally during development or as a result of some diseases such as cancer, neurodegenerative disorders, autoimmune disease or viral infection (Thompson, 1995; Chowdhury et al., 2006; Elmore, 2007; Lancellotti et al., 2009). It is a gene-regulated process and the key element in it is a group of cytoplasmic proteins known as Bcl2 family (Thornberry and Lazebnik, 1998). The two main members of this family are Bcl-2 and Bax proteins which act in opposite directions where Bcl-2 and Bcl-xL could protect cells by inhibiting apoptosis, while Bax enhances apoptosis (Kroemer, 1997; Ashkenazi and Dixit, 1998). In this study, the expressions of Bcl-2 family members were assessed at mRNA level in order to investigate the induction of apoptosis in hearts of electrocuted rats and the results showed that electrocution significantly down-regulate the Bcl-2, Bcl-xl expressions compared to control. Bcl-2 was affected by both type of death and time of sampling while Bcl-xl was affected by only cause of death. On the other hand, the only significant up-regulation in Bax expression was observed in E(1h) group compared to all other groups. On the line with our findings, Badawy et al. (2015) stated that apoptosis was induced in heart of rats after exposure to low voltage electrocution expressed by increasing the immunostaining of apoptotic protein caspase 3. In a case of acute heart infarction studied by Misao et al. (1996), in human bcl2 was increased in saved myocytes to protect the cardiac cells from apoptosis during the early infarction phase. Bcl2 also decreased in acute ischemic rat hearts (Celkan et al., 2007). Pro-apototic members of Bcl-2 family, increased intracellular calcium and ROS generation were reported to be the main reasons for releasing of the apoptogenetic mediators from mitochondria (Friedlander, 2003) and this agrees with our findings and explain them.
Regarding the histopathological findings in the heart tissue of electrocuted rats obtained in this study, interstitial hemorrhage was observed in some areas. Appearance of necrosis and fragmentation in some cardiac myocytes was also seen. In addition, contractile bands and waviness of heart muscle were recorded. All these changes are totally in agreement to those of Tuttnauer et al. (2006) and Badawy et al. (2015). The histopathological alteration of heart tissue including myocardial cell segmentation and hyper contraction, ischemia and hemorrhage in addition to square nuclei after electrocution were also observed by Fineschi et al. (2007), Franchet et al. (2013) and Viswakanth and Shruthi (2015). These changes could be returned to the ability of electric current to induce vascular injury on heart muscle and interfere with oxygen and blood supply to the heart leading to infarction and ischemia (Hackel and Jennings, 1988). Electric current can directly cause damages the integrity and disturb the resting potential of cellular membrane consequently lead to influx of water and solutes, cellular edema and finally cell destruction (Leibovici et al., 1995). Additionally, exposure of the living cells to electricity can also trigger their necrosis or apoptosis through formation of pores (electroporation) and allowing influx of calcium into cytoplasm. Excess calcium as a result of ischemia can activate cell contraction leading to formation of contractile bands and necrosis as reported by Rodríguez-Sinovas et al. (2007). This comes on line with the results obtained in the present study concerning the effects of electrocution on LDH, CPK and MDA levels and the effect of sampling time on their release.
CONCLUSION
Taking together, it may be concluded that the electric current causes extensive damage and DNA fragmentation in cardiac muscle, in which apoptosis plays an important role especially the Bcl2 family possibly through down-regulation of the anti-apoptotic genes Bcl-2 and Bcl-xL expressions and up-regulation of the pro-apoptotic gene Bax. Moreover, some alternative methods such as biochemical analysis, histopathological examination and DNA fragmentation could be used to support the results of Bcl-2 family gene expression to confirm the electrocution as the principle cause of death either the samples taken immediately after death or early post-mortem.
ACKNOWLEDGEMENTS
All the authors of the manuscript thank and acknowledge their respective Universities and Institutes.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTION
Mayada Ragab Farag designed the study, analyzed the data, collected literatures and drafted the manuscript and Kuldeep Dhama reviewed and performed the final check.
REFERENCES