Advances in Animal and Veterinary Sciences
Research Article
Antibacterial Activity of Actinobacteria Isolated from Mangroves of Andaman and Nicobar Islands, India
Sumitha Gopalakrishnan1, Jai Sunder1*, Venu Sasidharan2, Sai Elangovan Subramanian2
1ICAR- Central Island Agricultural Research Institute, Garacharma, Andaman & Nicobar Islands, India; 2Pondicherry Central University,Brookshabad, Andaman & Nicobar Islands, India.
Abstract | Mangrove environment supports a rich and diverse group of microorganisms. These bacteria perform various ecologically important activities in these ecosystems like nitrogen fixation, methanogenesis etc. and also by the release of various kinds of compounds in the environment. In the present study, sediment samples from the three zones of mangrove ecosystems viz. proximal, middle and distal were examined for Actinobacteria. A total of 23 Actinobacterial colonies were isolated. The THBC varied between 24 + 0.82 X 106 in Proximal Zone to 50 + 1.7 x 106 in the distal zone in Burmanallah with an increasing trend in the THBC from proximal to the distal zone. While in Carbyn’s Cove, THBC ranged between 20 + 2.16 x 106 in the middle zone and 42 + 1.7 x 106 in the distal zone. An increase in the TAC towards sea was obtained in TAC from Nil in the distal zone to 4 + 1.7 X 106 in the proximal zone in Burmanallah and in Carbyn’s Cove, TAC ranged between 0.0006 + 0.0001 X 106 in the distal zone and 8 + 0.47 X 106 in the proximal zone. ANOSIM (R: 0.932) have shown that microbial community structure was not different between sites and zones at a significance level of 1.7% . This also confirmed the similarity seen in the MDS. The diversity is high in the proximal stations and in the distal zone of Carbyn’s Cove. The Actinobacteria viz. Micromonospora sp., Streptomyces aburaviensis, Streptomyces aurantiogriesius, Streptomyces aurofasciculus, Streptomyces flavoviridis and Nocardia astreoides. S. aurantiogriesius have shown the maximum activity with more than 10 mm zone of inhibition against Klebseilla sp., E. coli, V. cholera and S. flexheri. While S. aburaviensis have shown high activity against Klebseilla sp., E. coli and V. cholera. The other Streptomyces species like S. aurofasciculus and S. flavoviridis have shown medium activity against the pathogens. The present study could be drawn that marine environment yields a greater number of isolates per source that is comparable with that obtained from mangrove soils. On the other side, the number of isolates obtained from mangrove sediments is lower, particularly in the case of Actinobacteria. The present study also shows that the two study area Barmanallha and Carbyn’s Cove) of south Andaman have high potential Actinobacteria with bioactivity useful for identification of some novel compounds in future.
Keywords | Mangrove, South Andaman, Bioactivity, Micromonospora, Streptomyces, Nocardia
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 30, 2015; Accepted | May 11, 2016; Published | May 19, 2016
*Correspondence | Jai Sunder, ICAR- Central Island Agricultural Research Institute, Garacharma, Andaman & Nicobar Islands, India; Email: jaisunder@rediffmail.com
Citation | Gopalakrishnan S, Sunder J, Sasidharan V, Subramanian SE (2016). Antibacterial activity of actinobacteria isolated from mangroves of Andaman and Nicobar Islands, India. Adv. Anim. Vet. Sci. 4(5): 230-236.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.5.230.236
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Gopalakrishnan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Mangroves are coastal wetland forests mainly found at the intertidal zone of estuaries, back waters, deltas, creeks, lagoons and mudflats of tropical latitudes. According to the latest estimate by the Forest Survey of India (FSI, 2013), the total mangrove area is approximately 4,628 km2 in India, out of which, 604 km2 occurs in Andaman and Nicobar Islands (Damroy and Krishnan, 2005; Ragavan et al., 2015). Fertility of the mangrove sediment results from the microbial decomposition of organic matter and recycling of nutrients (Sahoo, 2009). The activities of bacteria include nitrogen fixation, methanogenesis, and production of antibiotics, enzymes and human pathogens. The mangrove environment is a potent source for the isolation of antibiotic producing Actinobacteria, the Gram positive bacteria with branched filaments. Among the microorganisms Actinobacteria gained special importance due to their capacity to produce bioactive secondary metabolites (Thenmozhi et al., 2012) and more recently Himalomycin A antibiotic was isolated from Actinomycetes (Flora et al., 2015). On the whole, the last 55 years have seen the discovery of more than 12,000 antibiotics, and Most of the bioactive compounds from actinobacteria sort into several major structural classes such as amino glycosides (e.g. streptomycin and kanamycin), ansamycins (e.g. rifampin), anthracyclines (e.g. doxorubicin), β-lactam (cephalo-sporins), macrolides (e.g. erythromycin) and tetracycline (Basavaraj et al., 2010). According to Gunasekaran and Thangavel (2013), the Bay of Bengal harbour rich source of biologically active actinobacteria which has the capacity to produce novel and effective secondary metabolites with antimicrobial functions.
The mangrove ecosystems are generally divided into three zones according to the proximity to the coast viz. Proximal (near to coast), Middle and Distal (near to terrestrial environment). It is a confirmed fact that these zones does differ each other in mangrove species composition, faunal diversity and other physico-chemical factors. In this study, the Actinobacteria from the sediments of two mangrove environments (Burmanallah and Carbyn’s Cove) of South Andaman region was carried out for their diversity and antibacterial activity of the Actinobacteria extract against six pathogens.
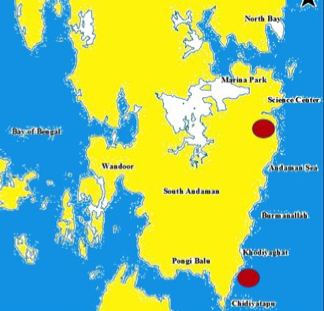
Figure 1: South Andaman map showing study area
MATERIALS AND METHODS
The sediment samples were collected aseptically from Proximal (near to coast), Middle and Distal (near to terrestrial environment) zones of mangrove ecosystems in Burmanallah and Carbyn’s Cove (Figure 1). Samples were air dried for 4 days at room temperature and 1 gm of dried sample was serially diluted in 50 % of 9 ml sterile seawater up to 10-6 dilution. From the dilution, 1 ml was inoculated into antibiotic incorporated Amphotericin (10 µL) and Nystatin (50 µL) starch casein agar medium by pour plate method and incubated at 28ºC for 7 to 9 days (Sahoo, 2009; Sivakumar et al., 2005). After the incubation, the appeared colonies were counted by using colony counter.
Morphologically different Actinobacteria were selected, identified to the species level based on the microscopic and biochemical test results (Sivakumar et al., 2005; Nonomura, 1974). Morphological observation of colony characteristics such as amount and colour of vegetative growth and the presence and colour of aerial mycelium and spores, and again the presence of diffusible pigments were recorded for each isolate and studied colonial growth on agar plate (Labede et al., 1985). The colonies isolated were purified by streaking method and then incubated at 28ºC for 6 days. The identification was done morphologically and biochemically (Tamura et al., 2011; Nonomura, 1974; Sherling and Gotileb 1966). The cultures were grown on ISP medium 2, ISP medium 3, ISP medium 4, ISP medium 5 and ISP medium 7 (Tyrosine Agar) as described by Sherling and Gotileb (1966) and El-Habib and Magda (2012) to examine the pigment production. The isolates were inoculated in Marine Actinobacteria growth (MAG) broth and incubated for 4-5 days and observed for aerial mycelium under 100x light microscope. Utilization of carbohydrates, salt tolerance 0 to 20% (w/v) NaCl and temperature tolerance along with urease activity, oxidase test, catalase test, protease, lipase, amylase, chitinase and cellulase activity were also tested (El-Habib and Magda, 2012; Gopalakrishnan et al., 2014).
The purified Actinobacterial strains obtained were tested for antibacterial activity against the human pathogens (Streptococcus sp., Klebsiella sp., Pseudomonas sp., E. coli, Vibrio cholera and Shigella flexnerii) by disc diffusion method (Badji et al., 2006; Alimuddin et al., 2011). Three Actinobacteria strains were selected based on their preliminary screening for antibacterial activity against the pathogens and were inoculated in MAG broth and incubated at room temperature for seven days. The culture broths were centrifuged at 8000 rpm for 10 minutes, and supernatant were used to test antibacterial activity. The pathogens were inoculated into nutrient agar medium by spread plate method. Filter paper discs were dipped in the centrifuged culture broth and placed on the plates by using sterile forceps and incubated at room temperature for 24-48 hours. The diameter of the zones of complete inhibition was measured. The results were statistically analysed using Microsoft Excel and PRIMER v7 software.
RESULTS and DISCUSSION
The total heterotrophic bacterial count (THBC) varied between 24 + 0.82 X 106 in Proximal Zone to 50 + 1.7 X 106 in the distal zone in Burmanallah (Table 1) which have shown an increasing trend in the THBC from proximal to the distal zone. While in Carbyn’s Cove, THBC ranged between 20 + 2.16 X 106 in the middle zone and 42 + 1.7 X 106 in the distal zone. The Total Actinobacterial Count (TAC) varied between Nil in the distal zone to 4 + 1.7 X 106 in the proximal zone. A similar result, an increase in the TAC towards sea was obtained in Carbyn’s Cove, where the TAC ranged between 0.0006 + 0.0001 X 106 in the distal zone and 8 + 0.47 X 106 in the proximal zone.
Table 1: Total Heterotrophic Bacterial Count (THBC) and Total Actinobacterial Count (TAC) from three zones of mangroves
|
Station |
Proximal |
Middle |
Distal |
|||
|
(cfu/ml) |
(cfu/ml) |
(cfu/ml) |
||||
|
THBC (x 106) |
TAC (x 106) |
THBC (x 106) |
TAC (x 106) |
THBC (x 106) |
TAC (x 106) |
|
|
Burma- nallah |
35 + 2.16 |
4 + 1.7 |
20 + 2.16 |
3 + 0.94 |
42 + 1.7 |
0 |
|
Carbyn's Cove |
24 + 0.82 |
8 + 0.47 |
36 + 0.47 |
2 + 0.82 |
50 + 1.7 |
0.0006 +0.0001 |
Identification of Actinobacteria
23 colonies of actinobacteria were isolated from the sediment samples during the present study. Isolated Actinobacteria were identified as Micromonospora sp. (S1), Streptomyces aburaviensis (S2), Streptomyces aurantiogriesius (S3), Streptomyces aurofasciculus (S4), Streptomyces flavoviridis (S5), Nocardia astreoides (S6) Bifidobacteria spp. (S7), Arthrobacter spp. (S8) and Pseudonocardia spp. (S9).
Streptomyces spp. were the most prominent actinobacteria isolated from the mangrove environment during the present study (Table 2). The other genera represented by Micromonospora, Nocardia, Pseudonocardia, Bifidobacteria and Arthrobacter were few in number. Carbyn’s Cove was found to harbour more number of actinobacterial species than Burmanallah. The proximal zone was found to be more diverse than the other zones in both the stations while no actinobacteria could be isolated from the distal zone of Burmanallah.
is completely different from other stations, Carbyn’s Cove and Burmanallah Middle Zones have shown more similarity than other stations (Figure 2). Also the proximal zones have also shown similarity.
According to ANOSIM (R: 0.932), microbial community structure was not different between sites and zones at a significance level of 1.7% (Figure 3). This also confirmed the similarity seen in the MDS.
It is evident from the diversity indices that the diversity is high in the proximal stations and in the distal zone of Carbyn’s Cove (Table 3). The evenness is more or less similar in all zones but dominance is high in distal and proximal zone of Carbyn’s Cove.
Carbyn’s Cove beach is one of the important tourist places in the Andaman Island with one side of the area covered by lush of mangroves and the other side rocky. The organic matter rich sediments by the way of influxes from land through the fresh water stream and by the way of high human interference in the proximal zone make it more productive. In Burmanallah, anthropogenic interventions were found to be much less than Carbyn’s Cove. Actinobacteria were found to dominate in sediments where organic load is more (Udotong et al., 2008; Dastager and Damare, 2013). So the more isolates from Carbyn’s cove can be attributed by more anthropogenic activities in this mangrove area.
Table 2: Occurrence of Actinobacteria in various zones along the mangroves
|
Actinobacteria |
CCP (X 106) |
BRP (X 106) |
CCM (X 106) |
BRM (X 106) |
CCD (X 102) |
BRD (X 102) |
|
Micromonospora sp. |
0 |
0 |
1 + 0.82 |
1 + 1.4 |
1 + 0.82 |
0 |
|
Streptomyces aburaviensis |
1 |
0 |
0 |
0 |
1 |
0 |
|
Streptomyces aurantiogriesius |
1 + 0.82 |
1 + 0.82 |
0 |
0 |
1 + 0.82 |
0 |
|
Streptomyces aurofasciculus |
2 + 0.82 |
1 + 0.82 |
0 |
0 |
1 + 0.82 |
0 |
|
Streptomyces flavoviridis |
1 |
0 |
1 + 0.82 |
1 + 0.82 |
1 |
0 |
|
Nocardia astreoides |
1 + 0.82 |
0 |
0 |
0 |
0 |
0 |
|
Bifidobacteria spp. |
1 + 0.82 |
1 + 0.47 |
0 |
0 |
0 |
0 |
|
Arthrobacter spp. |
1 |
1 + 1.4 |
0 |
0 |
0 |
0 |
|
Pseudonocardia spp. |
0 |
0 |
1 + 1.4 |
1 + 1.4 |
0 |
*CCP: Carbyn’s Cove Proximal zone; CCM: Carbyn’s Cove Middle Zone; CCD; Carbyn’s Cove Distal zone; BRP; Burmanallah Proximal Zone; BRM; Burmanallah Middle Zone; BRD; Burmanallah Distal Zone
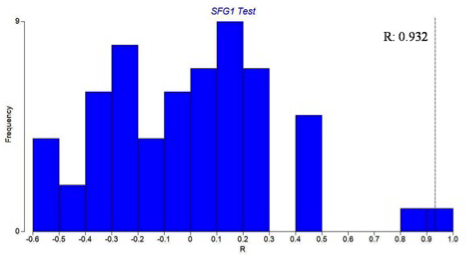
Figure 2: Similarity between the proximal, middle and the distal mangrove zones in Carbyn's Cove and group average
Table 3: Actinobacterial diversity in various zones in Carbyn’s Cove and Burmanallah
|
STATION |
S |
d |
J |
Brillouin |
Fisher |
H (log 2) |
1-Lambda |
|
CCP |
7 |
1.09956 |
0.9956 |
1.828 |
1.357 |
2.795 |
0.8581 |
|
BRP |
4 |
0.6198 |
1 |
1.274 |
0.7864 |
2 |
0.756 |
|
CCM |
2 |
0.2411 |
1 |
0.6015 |
0.3932 |
1 |
0.508 |
|
BRM |
3 |
0.4393 |
1 |
1.012 |
0.5898 |
1.585 |
0.6738 |
|
CCD |
6 |
2.791 |
1 |
1.097 |
- |
2.585 |
1 |
|
BRD |
0 |
- |
- |
- |
- |
0 |
- |

Figure 3: Microbial community structure in the mangrove zones: Permutation distribution of the test statistic Rshowing sloughing of villli and heterophillic infiltration)
According to Gunasekaran and Thangavel (2013), the Bay of Bengal may harbor rich source of biologically active actinobacteria which has the capacity to produce novel and effective secondary metabolites with antimicrobial functions.
Antimicrobial Activity
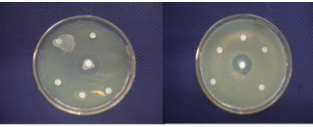
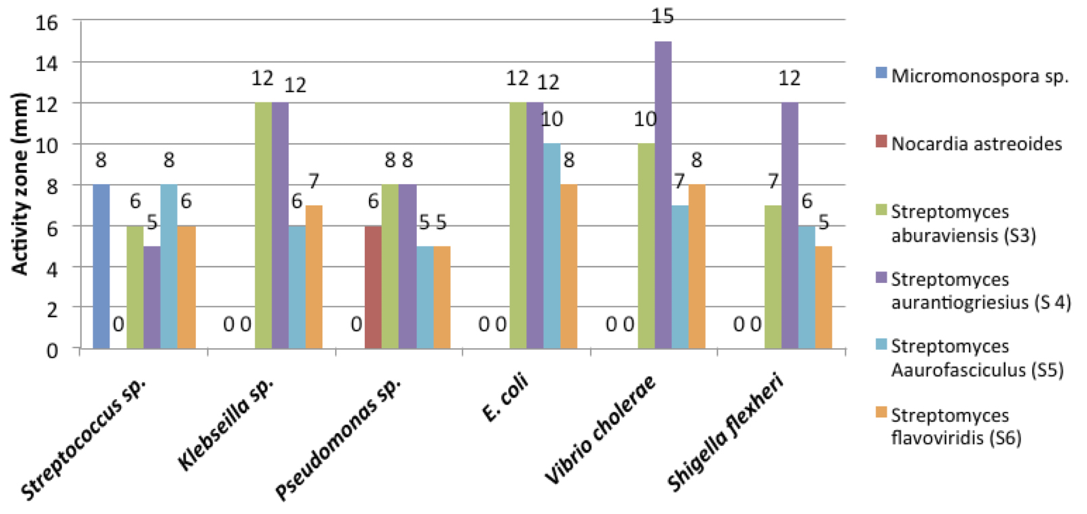
New drugs, notably antibiotics, are urgently needed to arrest and reverse the relentless spread of antibiotic resistant pathogens causing life threatening infections and risk which are undermining the viability of health-care systems all around the world (Talbot et al., 2006). During the present study, six morphologically unlike isolates, which showed antibacterial activity against various human pathogens, were selected and identified as Micromonospora sp. (S1), Streptomyces aburaviensis (S2), Streptomyces aurantiogriesius (S3), Streptomyces aurofasciculus (S4), Streptomyces flavoviridis (S5) and Nocardia astreoides (S6). Among these strains, S. aburaviensis (S2), S. aurantiogriesius (S3), S. aurofasciculus (S4) and S. flavoviridis (S5) have shown good inhibition zone (Figure 4) against various human pathogens.
Filamentous bacteria belonging to the order Actinomycetales, especially Micromomospora and Streptomyces strains, have a unique and proven capacity to produce novel antibiotics (Bentley et al., 2002), hence, the continued interest in screening such organisms for new bioactive and it is also becoming increasingly clear that un and under-explored habitats, such as desert biomes and marine ecosystems, are a very rich source of novel actinobacteria which have the capacity to produce interesting new bioactive compounds, including antibiotics (Jeffrey et al., 2007; Hong et al., 2009). Micromonospora sp. inhibited the growth of Streptococcos sp. While Nocardia asteroides have shown antibacterial activity against Pseudomonas sp. only. While all Streptomycetes have shown high activity against all the pathogens tested. It could be seen that S. aurantiogriesius (S 4) have shown the maximum activity with more than 10 mm zone of inhibition against Klebseilla sp., E. coli, V. cholera and S. flexheri, where Ampicillin antibiotic disc has shown maximum of 10 mm clearing zone (Figure 5).
While S. aburaviensis (S3) have shown high activity against Klebseilla sp., E. coli and V. cholera,where Ampicillin disc is used as standard. The other Streptomyces species like S. aurofasciculus (S5) and S. flavoviridis (S6) have shown medium activity against the pathogens. The most important observation was that all Streptomyces species have shown higher activity against Klebseilla sp., E. coli and V. cholerae. Micromonospora sp. and S. aurofasciculus have shown the highest activity against Streptococcus sp. While S. aburaviensis and S. aurantiogriesius have shown highest activity against all other pathogens. In each experiment Gentamycin 10mg (Himedia) and Ampicillin 10mg antibiotic discs (Himedia) were used as standards. Gentamycin has shown clearing zone maximum 22 mm for E.coli and Klebsiella sp. But for Streptococci, it has shown 5 mm clearing zone.
But Ampicillin has shown better result for all pathogens, by producing clearing zone of 10mm. All the Streptomyces sp. showed good activity above 10mm against E.coli sp. and Klebsiella sp., better than the antibiotic activity of Ampicillin.
A general conclusion could be drawn that marine environment yields a greater number of isolates per source that is comparable with that obtained from mangrove soils. On the other side, the number of isolates obtained from mangrove sediments is lower, particularly in the case of Actinobacteria. The present study shows that these isolated Actinobacterias strains, mostly Streptomycces spp. have shown antibacterial activity against the human pathogens and potential source of Actinobacteria from two-study area (Barmanallha and Carbyn’s Cove) of south Andaman. It can be concluded that mangrove ecosystems of South Andaman have high potential Actinobacteria with bioactivity useful for identification of some novel compounds in future.
acknowledgments
The authors Dr. Jai Sunder and Ms. Sumitha Gopalakrishnan thankfully acknowledge the funding received under the scheme “Women Scientist program (WOS-A) Department of Science and Technology, Government of India (DST No: SR/WOS-A/ LS-205/2008 Dated 28/08/2009)” and the facilities provided by the Director, CIARI for conducting this work. The authors Dr. S. Venu and Mr. Sai Elangovan, S. are thankful to Pondicherry University for providing facilities for conducting this work.
conflict of interests
The authors have no conflict of interest exist related to this manuscript.
authors’ contribution
Sumitha Gopalakrishnan and Sai Elangovan, Subramanian did the field sample collection and laboratory work. Dr. Jai Sunder and Dr. S. Venu guided these students and done part of the data analysis with bio-statistics and writing of the paper.
REFERENCEs