Advances in Animal and Veterinary Sciences
Case Report
A Case Report on Invasive Ductal Carcinoma in Dog
Tofazzal Md. Rakib1*, Md. Shafiqul Islam1, Mohammad Mahbubur Rahman1, Amam Zonaed Siddiki1, Bibek Chandra Sutradhar2, Mohammad Alamgir Hossain1, Md. Masuduzzaman1
1Department of Pathology and Parasitology; 2Department of Medicine and Surgery, Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh.
Abstract | Invasive ductal carcinoma is the most common invasive breast cancer in both women and pets, while the mammary tumors are the second most frequent neoplasm in dogs. A 5 years old bitch was presented at Teaching Veterinary Hospital of Chittagong Veterinary and Animal Sciences University, Bangladesh, having hard lump with irregular borders at post umbilical region. The biopsy sample was taken followed by histopathology and ER, HER2/neu, Ki67 immunostaining was done to know the type and cell origin of the tumour. Pleomorphism, increased mitotic figure and metastasis were revealed in histopathology. The section gave higher response to ER, HER2/neu Ig where response to Ki67 was low indicating luminal type A invasive ductal carcinoma. Grading tumours by immunostaining aid in prognosis and therapy.
Keywords | Invasive ductal carcinoma, Dog, ER, HER2/neu, Mammary gland
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 30, 2015; Revised | September 30, 2015; Accepted | October 01, 2015; Published | November 22, 2015
*Correspondence | Tofazzal Md. Rakib, Chittagong Veterinary and Animal Sciences University, Zakir Hossain Road, Khulshi-4225, Chittagong, Bangladesh; E-mail: rakibtofazzal367@gmail.com
Citation | Rakib TM, Islam MS, Rahman MM, Siddiki AZ, Sutradhar BC, Hossain MA, Masuduzzaman M (2016). A case report on invasive ductal carcinoma in dog. Adv. Anim. Vet. Sci. 4(1): 1-4.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.1.1.4
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Rakib et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Tumours of the mammary gland are among the most common neoplasms in the dog, but little is known about familial canine neoplasia. Some authors have reported a lower incidence in mixed breeds than in pure breeds. There have been no specific studies on the inheritance of canine mammary tumours (CMTs) but, as in human mammary cancer, most cases appear to be non-inherited (Stratmann et al., 2008).
Studies have shown that several non-neoplastic mammary intraepithelial lesions have been associated with the development of invasive mammary carcinomas. Intraepithelial lesions known for being precursors to mammary cancer are atypical hyperplasias, changes in atypical columnar cells and carcinomas in situ (Dabbs et al., 2006). Ductal carcinoma in situ (DCIS) is characterized by a proliferation of the malignant epithelial cells of the mammary ducts which tend to fill the ductal lumen, without disrupting the ductal basal membrane (Leonard and Swain, 2004).
Mutations in proto-oncogenes such as HER2 and EGFR, which are the two members of the family of epidermal growth factor receptors, are among the main genetic changes for the development of human cancer. The importance of these receptors has been extensively studied in human mammary neoplasms even originating treatment specific to inhibit its activity (Owens et al., 2004; Bhargava et al., 2005). HER-2 and EGFR overexpression was associated with malignancy in canine mammary tumors. However, in other studies no correlation between HER-2 expression and survival was found (Gama et al., 2009; Bertagnolli et al., 2011).
In 1974, the World Health Organization published the first ‘‘International Histological Classification of Tumours of Domestic Animals,’’ which included tumors and dysplasia of the mammary gland. This classification system was based on three seminal papers on canine malignant mammary tumor. Ductal carcinoma is a neoplasm that shows differentiation to interlobular ducts and is the malignant counterpart of the ductal adenoma. The neoplastic cell population is arranged in cords and tubules that surround slit like lumina that are often lined by a double layer of epithelial cells that exhibit significant anisokaryosis and anisocytosis; there are also numerous mitotic figures. Focal or multifocal areas of squamous differentiation and keratinization are present, with intracytoplasmic keratohyaline granules within some cells. The morphology of this neoplasm is identical to the apocrine ductal carcinoma of the skin (Goldschmidt et al., 2011).
Unlike laboratory rodents, dogs share a common environment with people and, therefore, may be exposed to some of the same carcinogens. Importantly, dogs with mammary adenocarcinoma are appropriate subjects for the study of breast cancer because the mammary gland is the most common site of neoplasia in female dogs (Vail and Macewen, 2000) and dysplasias develop before tumors in canine mammary tissue (Misdorp, 2002). Estimates of annual incidence of canine mammary neoplasia depend on the population studied and vary widely from 198 (Schneider, 1970) or 205 per 1,00,000 dogs (Dobson et al., 2002) to 111 per 10,000 female dogs between 3 and 10 years of age (Egenvall et al., 2005). The most commonly described biological characteristics of tumours viz., the histological types and grades, and hormonal receptor expression have been similar across populations (Olopade et al., 2008).
As in humans, advancing age, progesterone treatment, obesity in early life and diet also increased the risk of mammary tumors in the dog (Alenza et al., 2000). A genetic alteration commonly found in human breast cancer is amplification of HER-2 genes. HER-2 protein expression has been used to predict patient response to treatment. Expression of HER-2/neu (c-erbB-2) in canine mammary carcinoma is similar to that in human breast carcinoma, suggesting a possible role in carcinogenesis and value as a prognostic indicator (Ross et al., 2004).
A five years old local female dog was presented in Shahedul Alam Quadery Teaching Veterinary Hospital (SAQTVH) of Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh. The bitch had tumorous cutaneous outgrowth at post umbilical area. The owner mentioned it was seen by him 2months back and became larger then. The routine examination of blood was done and decision was made to excise the overgrowth. The excised tissue was transferred to Dept. of Pathology and Parasitology for histopathology and immunostaining.
Tissue sections were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4-μm thickness and stained with hematoxylin and eosin. The stained sections were examined under light microscope to get definite diagnosis of the lesions and its type. Typing and grading were done as per WHO (Hampe and Misdorp, 1974).
Sections were cut at 3µm thickness, mounted on to slides deparaffinised and rehydrated. Antigen retrieval was achieved by heat retrieval. Slides was then incubated with 100-200µl of primary antibodies for 30 min at room temperature in a moisture chamber, then rinsed in Phosphate Buffered Saline (PBS). Then the slides were incubated with secondary antibodies (Dako Label Polymer). Finally, Horseradish Peroxidase (Dako) was added to produce the characteristic brown stain. The sections were then counterstained, dehydrated and mounted for analysis. For each run of staining, a positive and negative control slide was also prepared. The positive control slides were prepared from mammary carcinoma known to be positive for the antigen under study. The negative control slides were prepared from the same tissue block, but incubated with PBS instead of the primary antibody. The immunohistochemistry were evaluated at the Histology and Immunopathology Laboratory of Chittagong Veterinary and Animal Sciences University (Chittagong, Bangladesh) using following markers: Estrogen Receptor (ER), HER-2/neu and Ki-67. A semi-quantitate histochemical score was used to record results of immunostaining having both the proportion and intensity of stained cells.
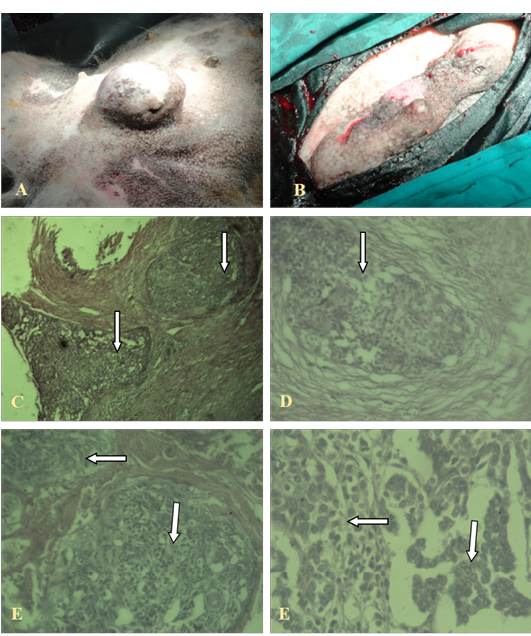
The invasive ductal carcinoma (IDC) was large (size, 16 cm) and affected several adjacent glands in continuity. There was some predilection for the posterior glands. In cases with prominent lymphatic permeation either one or both mammary chains were swollen and edematous. It was ill-defined and adherent to the skin and underlying tissues. Ulceration and necrosis was evident. IDC was characterized by a hard lump with irregular borders. The IDC lump felt harder, firmer and more anchored than a benign breast lump. The skin over the affected area was retracted inwards.
The neoplastic cells were hyperchromatic and pleomorphic and often polyhedral, with well-defined nuclei. The mitotic figures were also frequent. This carcinoma grew in a highly invasive fashion and lymphatic permeation was found. Necrosis was also common. Pleomorphism and the mitotic rate varied from low to high between sections. The amount of stroma was mostly small or moderate. Lymphocytes and plasma cells were found around, only rarely, in some sections. Blood vessels as well as lymphatics contained clusters of tumour cells as shown in Figure 1.
Immunostaining of the sections revealed proportion score (PS) 4 and intensity score (IS) 3 with total score (TS) 7 for ER; PS 3 and IS 3 with total score 6 for HER2. But in case of Ki67 immunostaining the sections revealed low response. So the immunostaining supports the case as invasive ductal carcinoma luminal type A which is shown in Figure 2.
As in humans and in contrast to mice and rats, spontaneous mammary tumors are the most common neoplasm of female dogs (Misdorp, 2002). Of the variety of methodologies
A, B: Gross cutaneous projection in post umbilical region with involvement of neighbouring glands; C, (4X), D (10X), E, F (40X): Showing pleomorphic cells with increased mitotic figures, without involvement of basal cells (in the arrows)
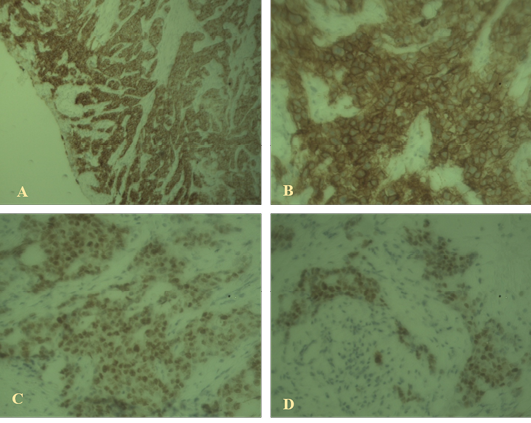
Figure 2: Immunohistochemical findings
A: High-grade ductal carcinoma in situ associated with invasive mammary carcinoma positive for estrogen receptor (40x); B: High-grade ductal carcinoma in situ positive for HER2 (100x); C, D: Weak staining with Ki67 indicating luminal A type invasive ductal carcinoma (40X).
available to evaluate HER2 status in breast cancers, the most convenient are immunohistochemistry (Jacobs et al., 2000). This case was subjected to relatively uniform tissue fixation and processing protocols. Our study used standard routine histologic staining and tumour was diagnosed according to grading system approved by WHO (Goldschmidt et al., 2011). In addition, our study used one of several commercially available ER, HER-2/neu, Ki67 antibodies used in clinical practice. With the set of antibodies chosen we aimed to identify features which are important in the malignant behaviour of the neoplasm. A high level of interlaboratory agreement in the assessment of ER, HER-2/neu, Ki67 immunostaining was achieved in the present study. Cellular features of DCIS determine the behaviour of this lesion (Freudenberg et al., 2009). He tested his assumption against an evolutionary theory, according to which a small-cell DCIS (DCIS1) becomes large-cell DCIS (DCIS3) before becoming invasive. He saw more grade I ductal cancer with a DCIS1 component and grade III ductal cancer with a DCIS3 component than vice versa. But he did not make any effort to distinguish at which point in the epithelial evolution from hyperplasia to atypical hyperplasia to DCIS the decision of the cells for infiltrating growth are made.
The cohort with pure DCIS showed a more ‘malignant pattern’ regarding all tumour markers, except for proliferation. High nuclear grade histology was defined according to the 1999 Consensus Conference of the Classification of DCIS (Schwartz et al., 2000), based on the presence of marked nuclear pleomorphism, large or very large nuclei and prominent nucleoli. Tumors with Allred score above 2 ER nuclear staining were classified as ER+ (Albain et al., 2010). Tumor HER2 membranous staining equivalent to 3+ intensity with Dako chromogen and staining more than 10% of cells was scored as overexpression. High Ki-67 index was defined as nuclear staining in more than 10% of tumor cells (Perou, 2011).
Mammary tumours, including mammary carcinomas, occur more often in the dog than in any other domestic species. The borderline between benign and malignant tumours is not always clear-cut histologically. Immunohistochemistry and microarray diagnosis of tumours can explain the specific cell origin and hormone involved which further helps in prognosis and therapy.
Acknowledgement
The author would like to thank Dr. Sharmin Chowdhury (Professor, Dept. of Pathology and Parasitology, CVASU) for providing financial support to complete the research work from HEQEP project (HEQEP CP: 3220); author would also like to thank Dr. Md. Zillur Rahman, Associate Professor, Department of Pathology, Chittagong Medical College, Bangladesh for providing technical support throughout the investigation process.
aUTHORS’ CONTRIBUTION
All the authors contributed equally.
Conflict of interest
There exisis no conflict of interest.
References