Advances in Animal and Veterinary Sciences
Review Article
Emerging and Re-Emerging Parasitic Zoonoses in India
Jyothimol G, Reghu Ravindran*
Department of Veterinary Parasitology, College of Veterinary and Animal Sciences, Pookode, Lakkidi P.O., Wayanad – 673576, Kerala, India.
Abstract | Parasitic zoonoses are considered as serious threat in a developing country like India, despite improvements achieved in the status of education and awareness of the people. This review provides an elaborate account of present status of emerging/re-emerging parasitic zoonoses in the country. Various important parasitic zoonoses viz., angiostrongylosis, cryptosporidiosis, cysticercosis, dirofilariosis, fasciolopsiosis, gnathostomosis, hydatidosis, leishmaniosis, toxoplasmosis, trypanosomosis and paragonimosis are discussed in detail. The parasitic infections like amoebiosis, babesiosis, capillariosis, clonorchiosis, diphyllobothriosis, dipylidiosis, sparganosis, trichinellosis, tick infestation and scabies are also discussed as they are increasingly reported in recent years.
Keywords | Parasites, Zoonoses, India, Emerging, Re-emerging
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 08, 2015; Revised | October 04, 2015; Accepted | October 05, 2015; Published | October 24, 2015
*Correspondence | Reghu Ravindran, College of Veterinary and Animal Sciences, Pookode, Lakkidi Wayanad – 673576, Kerala, India; Email: drreghuravi@yahoo.com
Citation | Jyothimol G, Ravindran R (2015). Emerging and re-emerging parasitic zoonoses in India. Adv. Anim. Vet. Sci. 3(12): 617-628.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.12.617.628
ISSN (Online) | 2307-8316;ISSN (Print) | 2309-3331
Copyright © 2015 Jyothimol and Ravindran. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
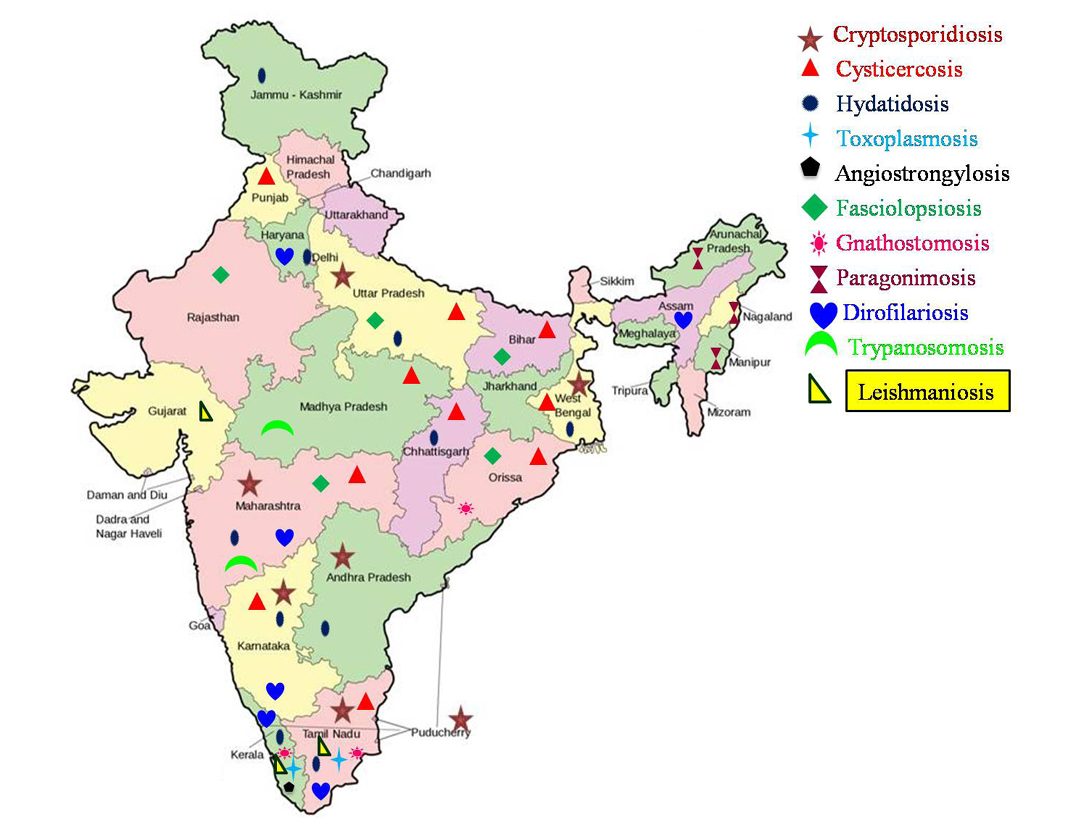
India is world’s seventh largest country with diversity in human culture and habits. Indian rural population sustain mainly on agriculture and livestock rearing. As per quinquennial livestock census, 2007; the total livestock population in India was 529.7 million, which included 37.6 per cent cattle, 19.9 per cent buffaloes, 13.5 per cent sheep, 26.5 per cent goats and 2.1 per cent pigs. The chances for occurrence of zoonotic diseases in rural community are high due to the lack of awareness on the etiology, sources and preventive measures of most of the zoonotic infections. Indian urban population, too are at the risk of zoonotic infections because of their intimate association with the pet animals. Other factors like poverty, lack of personal hygiene, open space defecation, reduced awareness on zoonotic diseases, scarcity of water, habit of eating raw/undercooked fish/meat, high population density, abundance of stray animals and vectors contributed to the rising prevalence of zoonoses in the country. In addition, cultural habits, natural/man-made calamities, population migration and population explosion also play an important role in the emergence/re-emergence of such conditions. Even though, many previous reviews are available (Chhabra and Pathak 2008a, b, c; Singh et al., 2010b), the present review provides an elaborate account of the occurrences of these emerging and re-emerging zoonotic parasitic diseases in the Indian subcontinent.
The disease conditions described in detail in the present review are classified based on the mode of transmission as direct transmission (fecal origin), indirect transmission (through intermediate hosts) and vector borne diseases (blood origin).
DISEASES OF FECAL ORIGIN
Cryptosporidiosis
Cryptosporidium parvum is one of the major causes of diarrhoeal disease in bovines and human beings especially children (Khubnani et al., 1997; Muraleedharan, 2009). It is one of the commonest parasites in Human Immunodeficiency Virus (HIV) patients and associated with high morbidity and mortality (Mohandas et al., 2002). Infection is self-limiting in immunocompetent hosts, but can be severe and persistent in the immunocompromised individuals such as Acquired Immune Deficiency Syndrome (AIDS) patients or malnourished children.
Cryptosporidiosis is considered as one of the emerging zoonoses in India due to increasing reports of the disease
from many parts of the country. Prevalence rate show variation in different regions of the country. Earlier reports suggest that northern states of the country like Delhi and Uttar Pradesh have prevalence rates of 3.5-4.3 per cent (Uppal and Natarajan, 1991; Ajjampur et al., 2010) and 7.2-11.6 per cent (Nath et al., 1999; Tahira et al., 2012) respectively in their human population. Studies conducted in South Indian states documented a prevalence of 3-7.6 per cent in Andhra Pradesh (Nagamani et al., 2001; Nagamani et al., 2007) and 2-35 per cent in Tamil Nadu (Muthusamy et al., 2006; Ajjampur et al., 2010). Anbazhagi et al. (2007) were able to detect Cryptosporidium oocysts in drinking water supplies of Chennai city of Tamil Nadu. Eastern states like West Bengal showed the prevalence of 6-33.3 per cent (Palit et al., 2005; Das et al., 2006; Gatei et al., 2007). In western states like Maharashtra, a prevalence of 1.36-5.6 per cent (Khubnani et al., 1997; Saraswati et al., 1998) was reported.
However, higher prevalence of cryptosporidial diarrhoea was reported from urban and slum areas of north-eastern states of the country (Ajjampur et al., 2008; Nath et al., 1999).There are only few reports on characterization of the organisms causing zoonotic human cryptosporidiosis. C. hominis, C. meleagridis and C. felis were characterized in pediatric patients and diarrhoeic individuals of Kolkata, West Bengal (Das et al., 2006; Gatei et al., 2007). In addition, C. hominis, C. parvum and C. bovis were detected in farm workers of West Bengal (Khan et al., 2010). C. muris was characterized from HIV patients of Vellore, Tamil Nadu (Muthusamy et al., 2006).
PCR based molecular characterization of bovine Cryptosporidium isolates from three different geographical areas of India (sub-temperate regions of Uttar Pradesh, eastern subtropical parts of West Bengal and Southern subtropical plains of Kerala, Andhra Pradesh and Karnataka) revealed the ubiquitous distribution of C. parvum (Paul et al., 2008). Similarly, C. parvum infection was diagnosed in 22.22 per cent of bovines in Bangalore, Karnataka (Veena et al., 2011) while 12.85 per cent of bovines revealed C. andersoni in three different geographical areas of India (Paul et al., 2009). Venu et al. (2012), reported C. andersoni, C. ryanae, C. parvum and C. bovis in bovines of South Indian states (Figure 1).
In bovines, prevalence rates of 10.89 per cent, 11.7 per cent, 9.05 per cent and 16.6 – 31.8 per cent were reported respectively from Maharashtra (Khubnani et al., 1997), West Bengal (Khan et al., 2010), Chennai (Prakash et al., 2009) and Andhra Pradesh (Nagamani et al., 2007; Bollam, 2005). In another study, out of 459 bovine dung samples collected, cryptosporidiosis was highest in Puducherry (86.67 per cent) followed by Karnataka (45.24per cent), Andhra Pradesh (41.33 per cent), Tamil Nadu (34.92 per cent) and Kerala (17.65per cent) (Venu et al., 2012).
Hence based on the available reports, the emergence of zoonotic cryptosporidiosis in human beings living in close association with livestock is increasing at an alarming rate.
Cysticercosis
Human cysticercosis is caused by metacestode stage (Cysticercus cellulosae) of Taenia solium. Neurocysticercosis (NCC), the most serious form of cysticercosis is seen in 60-90 per cent of human cysticercosis cases (Parija, 2004). NCC is the most common parasitic infection of Central Nervous System (CNS) which causes epilepsy (Gracia and Del-Brutto, 2003). The disease burden in the country vary greatly based on geographical area, religious rituals, food habits, personal hygiene, level of education and standard of living (Singh et al., 2010b). Incidence of cysticercosis is more in North Indian states like Bihar, Uttar Pradesh and Punjab (Prasad et al., 2008). Comparatively lower occurrence of NCC in Southern states may be attributed to the fact that, in South the use of raw vegetables as salads are rare compared to that practiced in North (Rajashekhar, 2004). However, recently there is an alarming increase in the case reports across the country from various states viz., Orissa (Sharma et al., 2011), Madhya Pradesh (Kinger et al., 2012), Maharashtra (Bothale et al., 2012), Uttar Pradesh (Yaquoob et al., 2009; Sharma et al., 2011; Parashari et al., 2012), Punjab (Bal et al., 2012), Manipur (Devi et al., 2007c), Chandigarh (Bhalla et al., 2008), West Bengal (Bandyopadhyay and Sen, 2009), Karnataka (Banu and Veena, 2011; Sathyanarayanan et al., 2011; Netravathi et al., 2011; Suchitha et al., 2012) and Tamil Nadu (Ratra et al., 2010).
In some states like Kerala and Jammu & Kashmir, case reports of human cysticercosis were very few. Jammu & Kashmir being a muslim majority state, pork consumption is prohibited on religious grounds. People of Kerala, with higher level of education and standards of hygiene (Singh et al., 2002), rear the pigs for meat consumption only on intensive system. Moreover, the pigs are not allowed for scavenging in open places in Kerala as observed in other states of the country.
Hydatidosis
Human hydatidosis is caused by the larval stage (metacestode) of the cestode parasite belonging to the genus Echinococcus. The two important species of this genus are E. granulosus and E. multilocularis which are associated with human hydatidosis causing cystic echinococcosis (CE) and alveolar echinococcosis (AE) respectively. This is endemic zoonosis where livestock is raised in association with dogs. Taori et al. (2004) reported that this human disease is endemic in central India. The annual incidence of hydatid disease per 1, 00,000 persons vary from 1 to 200 in India, (Parija, 2004). Hydatid disease in human beings mainly involves liver (75 per cent) and lungs (15 per cent). Numerous reports (Eckert et al., 2001) exist on the occurrence of human hydatidosis in almost all states of the country viz., Tamil Nadu (Vamsy et al., 1991), Chandigarh (Kanwar et al., 1992; Khurana et al., 2007; Rathod et al., 2011), Kashmir (Chishti and Ahanger, 1998), Delhi (Gupta et al., 2002), Uttar Pradesh (Pandey et al., 2007; Kumar and Hasan, 2008), Kolkata (Acharya and Gupta, 2009), Pune (Singh et al., 2010a), Haryana (Sing et al., 2010), Kerala (Anoop and Jabbar, 2010), Karnataka (Umesh et al., 2010), Maharashtra (Akther et al., 2011), West Bengal (Pathak et al., 2011), Andhra Pradesh (Faheem et al., 2013) and New Delhi (Kayal and Hussain, 2014). Akther et al. (2011) observed that the disease is common among house wives who have the practice of rearing sheep and goat.
Single report of hydatidosis due to E. multilocularis from India was in a man from Chandigarh (Aikat et al., 1978).
Diagnosis, prevention and control of hydatidosis are still at the juvenile stage, despite the pandemic occurrence of the disease. Usually the condition goes unnoticed until the cyst triggers some physical disabilities. Hence, there is an urgent need to create awareness among public and common people who are more prone to the condition.
Toxoplasmosis
Toxoplasma gondii is an obligate intracellular protozoan parasite of warm blooded animals and is one of the most common parasitic infections in humans. The condition is asymptomatic in immunocompetent humans, while can be fatal in immunocompromised adults and congenitally infected children (Tenter and Heckeroth, 2000). Toxoplasma encephalitis, a serious complication in immunocompromised individuals, especially in HIV patients has been previously reported from India (Chaddha et al., 1999). The prevalence of toxoplasmosis in India varies widely. The first national serological survey of T. gondii in India revealed the presence of serum Ig G and Ig M antibodies in 24.3 per cent and 2 per cent respectively out of 23,094 sera samples based on solid phase enzyme linked immunosorbent assay. In addition, seroprevalence rates were significantly higher in south than in north India (Dhumne et al., 2007). Based on Ig G ELISA, a seroprevalence of 13.14 per cent was observed in both immunocompetant and immunodefecient patients from Thirunelveli district of Tamil Nadu (Sucilathangam et al., 2012). A higher prevalence (20.3 per cent) of Toxoplasma antibodies in healthy voluntary blood donors from Karnataka was also reported (Sundar et al., 2007). In Kerala, a study using modified agglutination test (MAT) conducted among persons associated with veterinary clinics, Toxoplasma research lab, persons handling meat regularly and those rearing cats as pets at home and in women with history of abortion, a higher prevalence of 48 per cent was reported (Syamala et al., 2005).
Seroprevalence rates were significantly higher among women of low socioeconomic group compared to high (Yasodhara, 2004). Among pregnant women, higher risk was observed in first trimester. Those consuming boiled, unpasteurised milk and meat at least once in a week also had a positive correlation with prevalence rate (Pal et al., 2011). A higher prevalence of about 77 per cent was reported in women of reproductive age (Singh and Nautiyal, 1991). However, Mittal et al. (1995), based on IFAT reported only 7.72 per cent of Indian women of child bearing age with past obstetrical complications as seropositive. Highest repeated abortions were noted in women of more than 36 years of age. Prevalence studies especially in HIV patients and women with bad obstetrical history (BOH) were extensively conducted from different parts of the country (Meisheri et al., 1997; Mohan et al., 2002; Mittal et al., 1990; Sharma et al., 1997; Akoijam et al., 2002) indicating an alarming rise in the condition across the country. The seropositivity for IgG and IgM antibodies of Toxoplasma in antenatal women with BOH was 49.52 per cent compared to 12.38 per cent antenatal women with previous normal deliveries (Sarkar et al., 2012). Gupta et al. (2008) reported a case of Toxoplasma granuloma in a non - immunocompromised individual from Bangalore. A single case of cerebral toxoplasmosis in a pregnant non-immunocompromised patient from Thrissur, Kerala was also documented (Alapatt et al., 2009).
DISEASES TRANSMITTED THROUGH INTERMEDIATE HOSTS
Angiostrongylosis
The migration of larvae of Angiostrongylus cantonensis, the rat lung worm, is one of the reasons for eosinophilic meningitis in humans. The parasite has a worldwide distribution and show endemicity in Southeast Asian countries (Tsai et al., 2004). Human infections are attributed to the ingestion of intermediate host (snails and slugs) or paratenic host (crabs, frogs, fish, toads, monitor lizard) containing viable third stage larvae (Hochberg et al., 2007; Syed, 2001). Kerala is the only state in the country from where cases of eosinophilic meningitis due to larval migration of A. cantonensis were reported (Panackel et al., 2006). These authors documented five cases of the disease in persons of age 28-35 during January, 2000 – August, 2004. Later, Parameswaran, (2006) also documented a series of ten cases during February, 2004 to June, 2006 from Vaikom, Kerala.
In addition, A. cantonensis can cause ocular larva migrans (OLM) in human beings (Stephen et al., 2008). The combined occurrence of OLM and eosinophilic meningitis was also recorded (Baheti et al., 2008). These reports also came from Kerala.
There is a misbelief among some people of Kerala that the flesh of monitor lizards has rejuvenating / aphrodisiac properties. Hence, they go for consuming uncooked meat (mainly tongue and liver) of monitor lizard sandwiched between bread or banana slices, predisposing themselves to this condition. In majority of cases, the consumption of meat occurred after alcohol consumption.
Fasciolopsiosis
Human fasciolopsiosis is caused by a zoonotic trematode, Fasciolopsis buski, which usually inhabits the small intestine of humans and pigs. Humans acquire the infection by consumption of raw fresh water plants contaminated with metacercariae of the parasite. It is an emerging zoonosis in Southeast Asian countries like China, Taiwan, India, Bangladesh and Thailand (Bhattacharjee et al., 2009). Early reports indicate the prevalence of the disease varies in northern states of the country viz., eastern Uttar Pradesh (22.4 per cent) to Assam and eastern Bengal (60 per cent) (Chandra, 1984). However, the disease was reported from south India including Maharashtra (Shah et al., 1966; Manjarumkar and Shah, 1972). Recent reports indicate that the disease is confined only to eastern and northern Indian states like Uttar Pradesh (Bhatti et al., 2000; Bhattacharjee et al., 2009; Muralidhar et al., 2000; Singh et al., 2011), Bihar (Gupta et al., 1999; Kumari et al., 2006; Karthikeyan et al., 2013; Sunil et al., 2014), Rajasthan (Mahajan et al., 2010) and Orissa (Mohanty et al., 2012).
Gnathostomosis
Gnathostomosis is a very rare zoonotic infection in humans (Tiwari et al., 2009), which is caused by ingestion of improperly cooked meat of fish or frog that harbour the immature third stage larvae (Soulsby, 1982) of Gnathostoma spinigerum. Humans act as paratenic host. Hence, the larvae will migrate either subcutaneously or in visceral organs in humans and do not develop to mature stage (Pillai et al., 2011). Only 12 human cases were reported prior to 1994 from the country. In 1994, two more cases were reported from Madras, Tamil Nadu (Biswas et al., 1994). Recently, cases were reported from Orissa (Tiwari et al., 2009) and Kerala (Pillai et al., 2011). Even though, the condition is a rare clinical entity in the current scenario, it has the potential to emerge as zoonosis in near future.
Paragonimosis
Paragonimosis is an important food – borne zoonotic parasitic disease which is usually misdiagnosed as pulmonary tuberculosis. The condition is caused by parasite of the genus Paragonimus, the major species being P. westermanii and P. heterotremus. Metacercariae of the parasite were found encysted in the muscles of crabs and cray fishes. The practice of eating raw/undercooked crab/cray fish transmits the disease to human beings. Ingestion of raw/undercooked pork can also transmit the disease as they can act as a paratenic host for the parasite (Meehan et al., 2002).
Even though the condition was widespread and common among wild mammals in India, there was no scientific search on this topic till 1990’s. It is documented that most of the Asian and African countries are endemic for the parasite, where some of the cultural taboos foster human transmission (Mukae et al., 2001). In India, many of the human paragonimosis cases were reported from North Eastern states of the country and hence these states were considered to be endemic for the disease. The first human case was reported from Manipur in 1981 (Singh et al., 1982, 1993, 2004, 2005; Singh and Vashum, 1994), followed by another report from Maharashtra in 1984 (Patil et al., 1984). Later, several cases were reported from Manipur itself (Singh et al., 1986). Singh et al. (1992) reviewed 45 cases of different forms of paragonimosis in 1to 15 year old children from Manipur. A parasitological and immunological survey conducted in Arunachal Pradesh revealed that certain part of the state was hyperendemic for paragonimosis. The survey also reported sputum egg positivity of 20.9% and 4.1% in children (age </=15 years) and adults (age >15 years), respectively. Antibody positivity against excretory-secretory antigen of the adult worm in children and adults was 51.7% and 18.7%, respectively (Devi et al., 2007b).The first cerebral paragonimosis case from India was reported in an 8 year old boy from Nagaland (Singh et al., 2011). All these reports confirm the endemicity of paragonimosis in NE states of India.
The endemicity of paragonimosis in NE states can be explained in terms of low economic development, poverty, lack of education and poor access to health care facilities as they reside mainly in the hilly forested areas (Narain et al., 2015). Proper treatment and health education of the population can considerably reduce the prevalence of the condition in endemic areas.
VECTOR-BORNE DISEASES OF BLOOD ORIGIN
Dirofilariosis
Dirofilariosis is a mosquito transmitted filarial infection caused by nematodes of the genus Dirofilaria. Globally, the most common species identified in human infections is Dirofilaria immitis (Shobha et al., 2001). Dirofilariosis can be manifested in several forms like pulmonary (D. immitis), subcutaneous (D. immitis), or occular (D. repens) depending on the location of the parasite (Dam and Das, 2006). Human dirofilariosis cases were reported from various Indian states like Kerala (Ittyerah and Mallik, 2004), Tamil Nadu (Sathyan et al., 2006), Karnataka (Shobha et al., 2001; Karnaker et al., 2009), Assam (Nath et al., 2010) and Maharashtra (Khurana et al., 2010). The incidence is more common in areas with high mosquito density, warm climate and also in places where there is higher chance for contact with infected cats and dogs (Joseph et al., 2011). Other species like D. repens, D. tenuis and D. ursi were also reported (Joseph et al., 2011) as causative agent for human dirofilariasis around the world.
Subcutaneous cases caused by D. repens were also reported in literature. After the report of first case of subcutaneous dirofilariosis from Mumbai (Badhe and Sane, 1989) series of reports on the same condition were available from different south Indian states like Tamil Nadu (Padmaja et al., 2005), Karnataka (Khurana et al., 2010; Vaidya and Srikar, 2012), Maharashtra (Khurana et al., 2010) and Kerala (Joseph et al., 2011; Permi et al., 2011).
Cases of occular dirofilariosis were reported first from Kerala (Joseph et al., 1976; George and Kurian, 1978). In these reports, the causative agent was recorded as D. conjunctivae. This may be a misdiagnosis and the real causative agent might be D. repens. Later, large number of ocular cases due to D. repens were documented from different parts of Kerala (Sekhar et al.,2000; Mallick and Ittyerah, 2003; Sabu et al.,2005; Smitha et al., 2008), Karnataka (Nadgir et al., 2001; Kotigadde et al., 2012), Tamil Nadu (Sathyan et al., 2006; Mukherjee et al., 2012), Assam (Nath et al., 2010) and Haryana (Gautam et al., 2002).
Leishmaniosis
Leishmaniosis is a vector transmitted zoonotic disease caused by an intracellular protozoan parasite of the genus Leishmania. The disease is manifested either in cutaneous or visceral form (Soulsby, 1982). Phlebotomus sp. is the obligatory vector for the condition, of which P. sergenti is the only proven vector in India (Singh et al., 2006). Northeastern states of India like Bihar and West Bengal are endemic for visceral leishmaniosis (Chhabra and Pathak, 2008b). Sporadic cases were reported from Gujarath, Tamil Nadu and Kerala (Munshi et al., 1972; Kesavan et al., 2003). A recent news report said that visceral leishmaniosis was confirmed in a patient from Thrissur district, Kerala, who was a migrant labourer from Bihar/Uttar Pradesh where the disease is endemic (The HINDU, 23rd June, 2015).
Cutaneous leishmaniosis (CL) was reported from different parts of the country mainly from desert and semi-desert
Table 1: List of other zoonotic parasitic diseases
|
Disease |
Parasite |
State/area involved |
Reference |
|
Amoebiosis |
Entamoeba histolytica |
Karnataka |
Vijayshankar et al., 2010 |
|
Kerala |
Satish et al., 2012 |
||
|
Babesiosis |
Babesia sp. |
Baroda, Gujarath |
Marathe et al., 2005 |
|
Capillariasis |
Capillaria philippinensis |
Vellore, Tamil Nadu |
Kang et al., 1994 |
|
Chandigarh,Punjab |
Rana et al., 2009 |
||
|
Andhra Pradesh |
Vasantha et al., 2012 |
||
|
Cercarial dermatitis |
Madhya Pradesh |
Agarwal et al., 2000 |
|
|
Madhya Pradesh |
Rao et al., 2007 |
||
|
Karnataka |
Muraleedharan, 2000 |
||
|
Clonorchiosis |
Clonorchis sinensis |
New Delhi |
Mirdha et al., 1998 |
|
Chandigarh |
Rana et al., 2007 |
||
|
Diphyllobothriosis |
Diphyllobothrium latum |
Pondicherry |
Devi et al., 2007a |
|
Diphyllobothrium spp. |
Karimnagar, Andhra Pradesh |
Ramana et al., 2011b |
|
|
Diphyllobothrium spp. |
Vellore, Tamil Nadu |
Pancharatnam et al., 1998 |
|
|
Dipylidiosis |
Dipylidium caninum |
Karimnagar, Andhra Pradesh |
Ramana et al., 2011a |
|
Dracontiosis |
Dracunculus medinensis |
Rajasthan |
Johnson and Joshi, 1982 |
|
Rajasthan |
Choubisa et al., 2010 |
||
|
Fasciolosis |
Fasciola hepatica |
Lucknow, U.P. |
Vatsal et al., 2006 |
|
Vellore,TamilNadu |
Ramachandran et al., 2012 |
||
|
Sparganosis |
Spirometra sp. |
Uttar Pradesh |
Duggal et al., 2011 |
|
Toxocarosis |
Toxocara sp. |
Srinagar, Jammu & Kashmir |
Fomda et al., 2007 and Dar et al., 2008 |
|
Trichinellosis |
Trichinella sp. |
Uttarakhand |
Sethi et al.,2010 |
|
Chandigarh |
Pebam et al., 2012 |
||
|
Ophthalmomyiais |
Oestrus ovis |
Pottaneri, Tamil Nadu |
Senthilvel et al., 2008 |
|
Human tick infestation |
Haemaphysalis spinigera |
Kerala |
Prakasan and Ramani, 2003 |
|
Dermacentor auratus |
Kerala |
Ajithkumar et al., 2012 |
|
|
Scabies |
Sarcoptes scabiei |
Gujarat, Haryana |
Pal and Dave, 2006 and Tikkaram et al., 1991 |
|
Notoedric scabies |
Notoedres cati |
Madhya Pradesh |
Chakrabarti, 1986 |
areas (Ajjampur et al., 2008). Recent reports indicate a tendency of spreading the condition to non-endemic areas especially towards southern states like Kerala. First report of CL in Kerala was in 1988 which was an imported case from Saudi Arabia (Lohidakshan et al., 1988). First indigenous case from Kerala was from Malappuram District (Muhammed et al., 1990). Bora et al. (1996) reported a prevalence rate of 2.7 per cent for CL in Nilambur division of Malappuram district, Kerala. Recently, CL was also reported from Kasargode (Kumaresan and Pramod, 2007), Thiruvananthapuram and Kollam (Simi et al., 2010) districts of Kerala.
Trypanosomosis
Trypanosomes are flagellated protozoan parasites infecting a wide range of animals and man (Soulsby, 1982). Previously, it was believed that human infections by animal species of Trypanosoma were impossible due to the presence of trypanolytic factor in human serum. However, T. evansi and T. congolense were demonstrated to be resistant to human plasma in certain conditions (Hawking, 1978). Even though, T. evansi is endemic in domestic animals in India, human cases of trypanosomosis were reported occasionally (Chhabra and Pathak, 2008b). Among the cases reported from the country so far, the causative species identified were T. evansi and T. lewisi.
The first Indian report of human trypanosomosis caused by T. evansi came from Maharashtra (Joshi et al., 2005). A serologic survey carried out in the affected area showed 4.5 per cent prevalence for the condition (Shegokar et al., 2006). Later, another case was also reported from the same state (Powar et al., 2006).
Human infections due to T. lewisi like organisms were reported from Madhya Pradesh (Shrivastava and Shrivastava, 1978), Maharashtra (Kaur et al., 2007; Banerjee et al., 2008; Shah et al., 2011) and New Delhi (Verma et al., 2011).
Various reports on the other zoonotic parasitic diseases are listed in Table 1.
CONCLUSIONS
The prevalence, emergence and re-emergence of various zoonotic parasitic diseases, are increasing in recent decades to an alarming level in India. Lack of proper surveillance and scarcity of information regarding the existence of asymptomatic animal carriers could be few reasons for underestimation of their prevalence. Climate change due to global warming, poverty, lack of personal hygiene, certain ritual taboos, increased population density, immunosuppresssion especially due to diseases like AIDS, presence of stray animals etc. have augmented the transmission of diseases from animals to human beings. Prevalence of most of the zoonotic diseases varies depending on geographical location, human culture and misbelieves / taboos followed in that region. However, the central and southern India reveals maximum reports of the zoonotic diseases compared to the other parts of the country. Proper reporting of such cases by physicians can thus help in understanding the background situation which will aid in control of such diseases. In addition, molecular epidemiological and spatial analytical tools are helpful for better understanding of the current status of such conditions. Health education, vector control, control of animal movements and improvement of socio-economic status of rural population can help to control parasitic zoonoses in the country. The need for better coordination of medical and veterinary personnel to formulate appropriate control strategies should also be considered in future.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
Authors are thankful to Indian Council of Agricultural Research (NAIP C-2066, NFBSFARA/BSA- 4004/2013-14) and Kerala State Council for Science, Technology and Environment (020/SRSAGR/2006/CSTE, 022/YIPB/KBC/2013/CSTE, 010-14/SARD/13/CSTE) for funding various research projects to the department.
AUTHORS CONTRIBUTION
Both authors contributed equally to the manuscript.
REFERENCES