Advances in Animal and Veterinary Sciences
Review Article
Camelpox and Buffalopox: Two Emerging and Re-emerging Orthopox Viral Diseases of India
Manimuthu Prabhu1*, Revanaiah Yogisharadhya2, Selvaraj Pavulraj3, Chinnathambi Suresh4, Gopal Sathish5, Raj Kumar Singh6
1Sheep Breeding Research Station, Tamil Nadu Veterinary and Animal Sciences University (TANUVAS), Sandynallah, The Nilgiris-643 237, Tamil Nadu, India; 2ICAR- National Institute of Veterinary Epidemiology and Disease Informatics (ICAR-NIVEDI), Bengaluru, Karnataka-560 024, India; 3Equine Pathology Laboratory, ICAR-National Research Centre on Equines (ICAR-NRCE), Hisar, Haryana-125001, India; 4Veterinary College and Research Institute (VCRI), TANUVAS, Ramayanpatti, Tirunelveli-627 358, Tamil Nadu, India; 5Veterinary Dispensary, Panchetti-601 204, Tamil Nadu, India; 6ICAR-Indian Veterinary Research Institute (ICAR-IVRI), Izatnagar, Bareilly-243 122, Uttar Pradesh, India.
Abstract | World Health Assembly declared that the world is free from smallpox virus infection and vaccination against smallpox is not recommended since May 8, 1980. Since then several incidences of infections due to poxvirus reported in different parts of the world in humans and animals and the trend has now been increasing. In India, camelpox and buffalopox are the two important Orthopoxvirus (OPV) infections considered as emerging public health concern since last decade due to augmented reports of outbreaks and isolated cases. Both are highly contagious zoonotic viral diseases. Camelpox is an economically important, notifiable skin disease of camelids and could be used as a potential bio-warfare agent. Though, vaccines are available in few countries, this disease has received much attention in present days due to emergence of infections in human beings. Buffalopox is also causing tangible and intangible losses in affected herds which has no commercial vaccines at field to protect. Hence, novel, specific, sensitive, rapid and cost-effective diagnostic techniques would be useful in identification, thereby early implementations of therapeutic and preventives measures to curtail these diseases. This review provides an overview on the epidemiology, clinical picture, biology, diagnostic approaches and the preventive measures on the two emerging and re-emerging disease of India viz. camelpox and buffalopox.
Keywords | Orthopox virus, Camelpox, Buffalopox, Zoonosis, Emerging disease
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 09, 2015; Revised | August 01, 2015; Accepted | August 02, 2015; Published | August 12, 2015
*Correspondence | Manimuthu Prabhu, Sheep Breeding Research Station, TANUVAS, Tamil Nadu, India; Email: drprabhuvirol@gmail.com
Citation | Prabhu M, Yogisharadhya R, Pavulraj S, Suresh C, Sathish G, Singh RK (2015). Camelpox and buffalopox: Two emerging and re-emerging orthopox viral diseases of India. Adv. Anim. Vet. Sci. 3(10): 527-541.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.10.527.541
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Prabhu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Since last two decades, several unexpected outbreaks and epidemics of disease caused by viruses has been reported in humans and animals (Howard and Fletcher, 2012). This is the main constraint of majority of the developing countries where identification and control of emerging and re-emerging diseases are difficult and origin of diseases have major concern. Most of the emerging and re-emerging diseases causing agents are of zoonotic significance (Woolhouse and Gowtage-Sequeria, 2005). Almost a new virus has been emerging every year since the last two decades of which the pox viral diseases are one of the most significant diseases. Since after eradication of smallpox infection, there are several reports on outbreaks of other poxvirus infections in humans (Singh et al., 2012). Initial outbreaks attributed to cowpox virus (CPXV) was recognized as caused by Vaccinia virus (VACV), species belongs to the genus Orthopoxvirus (OPV) (Van Regenmortel et al., 2000). Immunogenic similarity of Variola virus (VARV) with VACV helped in prevention smallpox infections in humans by mass immunization program executed by means of World Health Organization (WHO), resulted in eradication of smallpox from the earth in 1979 (Fang et al., 2006). Nevertheless, emergence of new VACV variants and bioterrorism represents key alarm and threat for more than half of the human populations of the world. Recent emergence of genetically related/allied OPVs has been reported all over the world viz. buffalopox, monkeypox and bovine vaccinia infections (Venkatesan et al., 2010a). Recently, OPV diseases outbreaks recorded more frequently in domestic animals as well as in humans (Shchelkunov, 2013), which reveals the presence of new condition in ecology and evolution of zoonotic OPVs. The pattern of evolution reminds that a VARV like viruses may emerge as zoonotic OPVs in due course of natural evolution process. Further, there is a persistent need for international control over the outbreaks in different countries of the world to provide a speedy reaction and prevention of outbreaks in developing/propagating epidemics. Camel populations in the world are estimated around 25.89 million, which spread around in 47 countries (FAOSTAT, 2012). Out of which, 85% camels resides in northern and eastern Africa and remaining populations are in Indian subcontinent and Middle East countries. India rank 7th in camel population of the world, and camel population is confined to north and western parts (Rajasthan, Haryana, Punjab and Gujarat states) of the country where 93.12% of Indian camel population inhabitates (Meena, 2014). In India, camel represents about 0.08% of the total livestock population (http://dahd.nic.in). The camelpox infection is reported in places where the presence of large populations of camels and the migration infected animals to new herds makes continues movement and persistence of virus in herds as possible (Fowler, 2010). The buffalo contributes around 21.23% of the total livestock population with the population of about 108.7 million buffaloes in the country as per 2012 Census (http://dahd.nic.in). There are no inhibitions for the possible re-occurrence of smallpox infections in humans in future due to natural evolution process of the currently circulating zoonotic OPVs. Continuously increasing zoonotic OPVs infections in the human populations in the absence of mass smallpox vaccination, increases the probability of emergence of new variants of viruses, which may be very dangerous for human life. Therefore this review focuses on two important OPVs existing in India – the camelpox and the buffalopox infections, particularly on their origin, epidemiology, clinical picture, diagnosis, preventive measures and global impact on veterinary and public health.
CAMELPOX
Camelpox is the most important acute contagious OPV disease of camelids (Camelus bactrianus and Camelus dromedarius) (Azwai et al., 1995). The disease was first reported in between 1893 and 1902 from Russia (Wernery and Kaaden, 2002), Rajaputana and Punjab parts of India (Leese, 1909) and later from different parts of the world (Figure 1) (Hafez et al., 1992). It is endemic in camel-rearing countries of Africa and Middle East Asia,
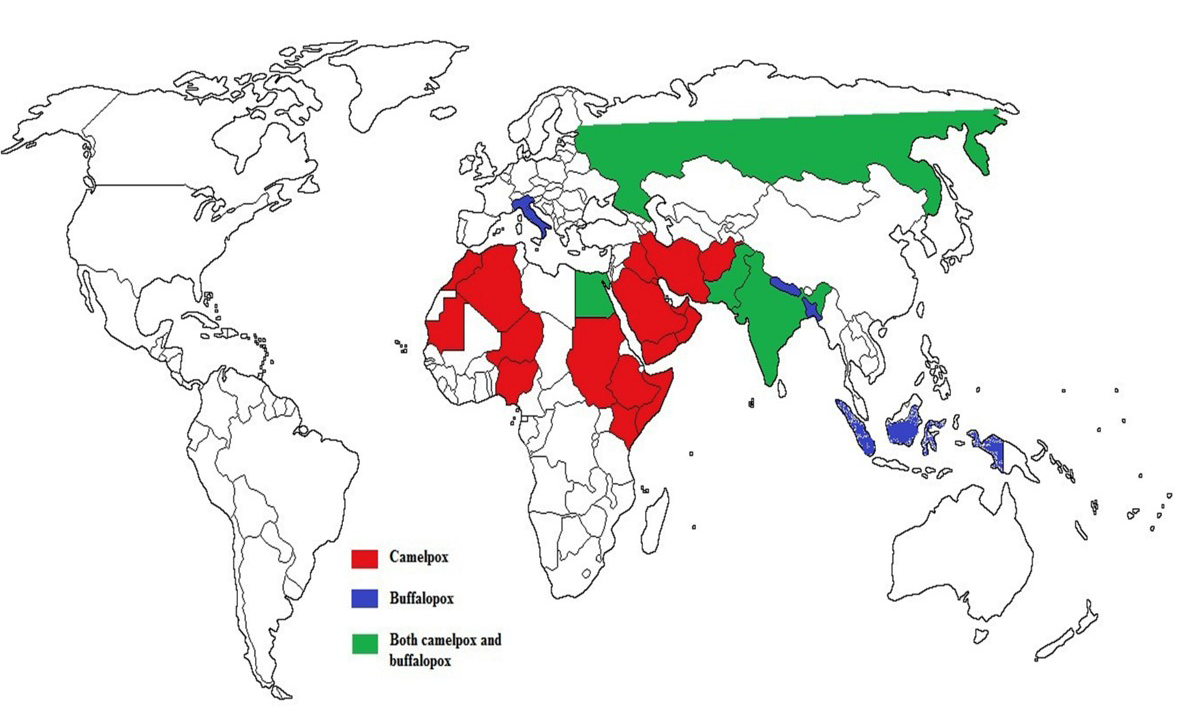
Figure 1: Epidemiology and geographic distribution of camelpox and buffalopox infection in the world
in which places camels are reared for milk and draught. It is economically significant disease of camels in terms of morbidity and mortality, weight loss and reduction in milk yield (Jezek et al., 1983). The disease commonly seen in younger camels, between 2 to 3 years of age and outbreak of disease is allied with poor nutrition and weaning. Clinically, camelpox infections may occur as any of three forms viz. severe, generalized milder, localized forms. Generalized and localized forms of infections mainly observed in young and older animals respectively. Morbidity, mortality and case fatality rates (CFR) may ranges from 30 to 90%, 1 to15% and 25% respectively (Al-Ziabi et al., 2007). The recovered animals may acquire life-long protective immunity to re-infection of homologues virus (Wernery and Kaaden, 2002).
Disease Classification
Camelpox is a notifiable skin disease of camelids as classified by Office International des Epizootics (World organization for animal health) (Elliot et al., 2008). Camelpox virus (CMLV) is primarily a disease of camels and so far infection in humans has not received much attention. Previously, the CMLV infection was thought to be a potential zoonotic agent to humans (Leese, 1909; Davies et al., 1975) as evidenced by smallpox-unimmunized individuals in Somalia (Kriz, 1982; Jezek et al., 1983). Recent reports indicate that CMLV can infect camel handlers under certain conditions (Bera et al., 2011).
Geographical Distribution
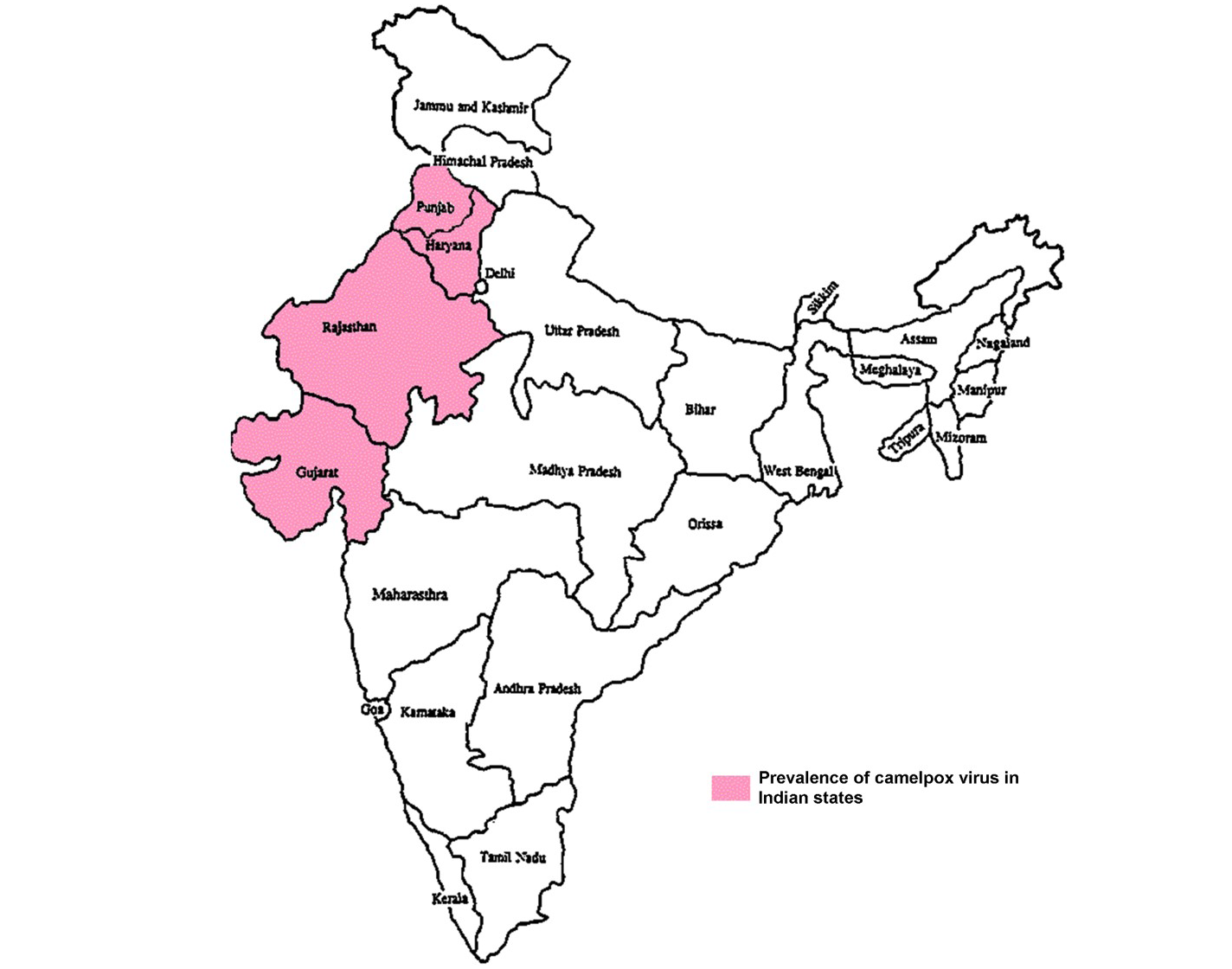
Even though, causative agent was not identified and isolated till 1969 (Sadykov, 1970), camelpox infection was a well-known disease in different parts of camel rearing countries (Figure 1) (Elliot et al., 2008). The infection is endemic in the Middle East countries (Iraq, Iran, Saudi Arabia, United Arab Emirates and Yemen), in Asia (India, Pakistan and Afghanistan) in Africa (Kenya, Egypt, Algeria, Somalia, Mauretania, Ethiopia, Oman, Sudan Nigeria and Morocco) and in the southern parts of former USSR (Chauhan and Kaushik, 1987; Hafez et al., 1992; Renner-Muller et al., 1995; Khanna et al., 1996; Marodam et al., 2006; Bhanuprakash et al., 2010a, 2010b; Duraffour et al., 2011; Balamurugan et al., 2013). Lately, the outbreaks were also recorded from Syrian provinces namely, Hama and Duma (Al-Ziabi et al., 2007) and in India (Figure 2) (Bhanuprakash et al., 2010a; Bera et al., 2011).
Etiological Agent
Camelpox is caused by CMLV, a linear double stranded DNA virus with a genome size of 205.719 kbp, belong the genus Orthopoxvirus (OPV), subfamily Chordopoxvirinae of Poxviridae family (ICTV, 2005). Initially it was considered as smallpox-like disease causing agent at the time of smallpox virus eradication (Baxby, 1972), later it lead to identification, isolation and characterization of CMLV. The average virion size is 224 × 389 nm. CMLV remains one of the neglected members of the OPVs till now. It is impossible to differentiate from the prototypic member of the family Poxviridae, Vaccinia virus (VACV) (Gubser and Smith, 2002; Sheik et al., 2009). Nevertheless, structure of the genome and DNA sequences for all open reading frames (ORFs) phylogenetic analysis revealed that CMLV is distinct from VACV and VARV (Afonso et al., 2002). But the DNA sequences of CMLV were most related to VARV (Gubser and Smith, 2002).
Physiochemical Properties of CMLV
CMLV is like any other OPVs, shows unpredictable responses to different physical/chemical agents. CMLV is resistant to ether and sensitive to chloroform (Tantawi et al., 1974; Davies et al., 1975). It is more sensitive to extreme ranges of acidic and alkaline conditions (Davies et al., 1975). The virus can be easily killed by boiling or autoclaving at least 10 min and few minutes in ultraviolet rays (Coetzer, 2004). The detailed difference in the physico-chemical properties of different CMLV strains has been reviewed earlier (Duraffour et al., 2011). CMLV (H 520 strain of Kenya) haemagglutinates cockerel erythrocytes (Davies et al., 1975). But, the 78 strain showed agglutination with chicken RBCs at room temperature (pH 6-8) with low titer (Al-Falluji et al., 1979). In general, pox virions show high environmental stability (tolerance to temperature, pH and chemicals) and may stay as contagious for several months (Rheinbaden et al., 2007).
Host Range
Naturally, CMLV has extremely narrow host range, which primarily affects camels along with mild infection in humans as per recent reports. Experimental inoculation of CMLV in sheep, goat, rabbit, guinea pig, rat, hamster and mice has not been successful so far in establishment of infection (Ramyar and Hessami, 1972; Bhanuprakash et al., 2010b). Apart from natural host, monkeys and suckling mice have been successfully infected experimentally (Baxby, 1972). In contact sheep and cattle with naturally infected camels do not acquire the infection. However, certain isolates of CMLV (CP/Nw/92/2) isolated from Sudan were able to induce local skin lesions in chickens (Khalafalla and Mohamed, 1998).
Disease Transmission
The disease is transmitted naturally by direct contact of infected animals with susceptible animal or by indirect contact with contaminated environment (Al-Ziabi et al., 2007). The route of transmission is via inhalation or through skin abrasions. The infected camels shed the virus in secretions like saliva, milk, ocular and nasal discharges (Ramyar and Hessami, 1972). The dried scab shed from the infected animals is rich in virus and is the potential source for environmental contamination for at least four months. Ticks population in rainy season is speculated to be involved in the spread of camelpox infection (Wernery et al., 1997b). Conversely, mosquitoes, biting flies and other vectors are also involved in transmission of the infection (Bhanuprakash et al., 2010a). Camelpox outbreaks were reported in isolated pockets in different parts of the world and isolated viruses of in different degree of virulence (Duraffour et al., 2011), which raises the question of wild animal as a reservoir of infection for CMLV other than camels. Further, outbreaks commonly seen during rainy seasons when rodents are active, so it is possible that, rodents may act as natural carriers of CMLV (Shchelkunov, 2013).
Environmental and other Predisposing Factors
Outbreaks occurs frequently in temporal regions due to continuous movement of camels for watering and grazing which leads to mixing of different herds and introduction of new animals into a herd (Azwai et al., 1996). Involvement of arthropod vector may also be the potential source which needs to be investigated. The other factors which influences the occurrence of the disease includes age, stress, nutritional status of the animals, season, migration of animals, presence of other diseases in incubation phase and of course the virulence of the virus (Davies et al., 1975; Jezek et al., 1983; Al Hendi et al., 1994).
Prevalence of Disease in Animals and Humans
The disease may cause both tangible and intangible losses due to morbidity and high mortality in young animals with weight loss, reduced milk production, draft power and trade constraint of infected camels and their by-products (Jezek et al., 1983). Morbidity may vary based on spread virus strain in particular herd. Serological surveys from several developing countries like Kenya, Sudan and Libya exposed the prevalence of high antibody level to camelpox virus infection (Wernery and Kaaden, 2002). The incidence rate and case fatality is more in males than females (Jezek et al., 1983) and the mortality is higher in young animals than adults (Kritz, 1982). The mortality may ranges from 10 to 28 % and 25 to 100 % in adult and young animals respectively (Bhanuprakash et al., 2010a). Current literature indicates the occurrence of mild zoonotic cases of infection in people who handle the infected animals (Bera et al., 2011).
Clinical Manifestations in Animals
The incubation period ranges from 4-15 days followed by pyrexia. Later, there will be papules formation on the lips, afterwards papules became vesicles, pustules and scabs formation (Bhanuprakash et al., 2010a). The disease occurs commonly as in-apparent form with mild infection limited to the skin, rarely severe systemic infections may appear, varying based on the strains of CMLV (Wernery and Kaaden, 2002). The infection is characterized by pyrexia, lymphadenopathy and typical pox lesions on skin (Wernery et al., 1997a). These skin lesions primarily may appear on the head, eyelids, nostrils, ears and in the severe cases swelling of whole head may be seen. In acute cases intense skin pruritis also observed. Later, the skin lesions extend to the neck, mammary glands, genitalia, limbs, and perineal region. The generalized form of lesions may appear in entire body and takes about 4 – 6 weeks to heal. In these cases, pox lesions may be seen on the mucous membranes of the mouth, digestive and respiratory tracts. Normally, CMLV infection is of benign type in adult camels. Nevertheless, severe forms infection causes loss of vision with increased CFR in young camels. Further, abortions, still birth, loss of weight with reduced milk production may also be seen in adults (Higggins et al., 1992; Kritz, 1982; Khalafalla and Mohamed, 1996; Hussien and Al-Mufarrej, 1999; Bhanuprakash et al., 2010a). Infected animals may show ptyalism and lacrimation with mucopurulent nasal discharge. Sometimes, systemic form of infection causes anorexia, diarrhea and death due to secondary bacterial complications and septicemia caused by Staphylococcus aureus ((Nothelfer et al., 1995; Wernery and Kaaden, 2002).
Zoonotic Significance
CMLV was found to be zoonotic in nature (Leese, 1909; Davies et al., 1975) with little evidence till recent days. At times, the virus could be pathogenic for man like cowpox and monkey pox (Marennikova et al., 1974). Study for the vulnerability of humans to camelpox virus infections was undertaken in Somalia in early 1980s. Kritz (1982) and Jezek et al. (1983) revealed that most of camel herdsmen never had smallpox vaccination. Skin rashes were observed in few herders, although clinical samples collected from affected individuals never revealed OPV, further transmission of camelpox was not reported. It could be one of the potential bio-warfare agents (Marennikova et al., 1974; Balamurugan et al., 2008). Immunocompromised humans were more susceptible to CMLV infections (De Clercq, 2002). The possibility of zoonotic transmission was not decisively confirmed till 2010, while the etiological agent was isolated from skin lesions and pustules on the hands of camel herders in Indian who were in contact with infected animals (Bera et al., 2011). The skin lesions remain localized without transmission between humans (Bray and Babiuk, 2011). The clinical manifestations in infected humans may include fever (101-102°F), itching, erythema along with formation of localized edema, papules, vesicles, ulceration and scab formation over fingers and hands. The lesions may be painful initially; later, skin vesicles may burst and became dry leaving scars formation (Bera et al., 2011).
Diagnostic Procedures
Numerous diagnostic methods have been developed for diagnosis of camelpox virus infection (Pfeffer et al., 1998). Tentative diagnosis for CMLV infection may be made based appearance clinical signs. Conversely, camels with mild clinical signs should be discriminated from other diseases like papilloma virus infections, contagious ecthyma (orf) and insect bites. Camelpox infections are usually diagnosed based on appearance of clinical signs, epizootiological, postmortem findings (Buchnev et al., 1987), virus isolation and identification, electron microscopy and antigen capture ELISA (Bhanuprakash et al., 2010a). Restriction enzyme analysis and transmission electron microscopy (typical brick-shaped) can be used for differentiation of parapoxviruses from other OPVs (Murphy et al., 1999; Al-Ziabi et al., 2007).
Virus Isolation
For isolation of camelpox virus, embryonated chicken eggs aged 11-13 days, primary cell culture and cell lines are used. When inoculated into the 11 to 13-day old embryonated chicken eggs through chorioallantoic membrane route (CAM) produces greyish white, dense, pock lesions on the surface of CAM after 5 days. In contrast, Vero cell line adapted viruses produces longer, opaque, white proliferative, tigroid pock lesion on CAM (Marodam et al., 2006). The cell lines like HeLa, MA-104, WISH, GMK-AH1, BSC-1, Vero, baby hamster kidney cells and MS monkey kidney cell lines can be used (Baxby, 1972). Primary cell cultures viz. lamb testis, lamb kidney, calf kidney, camel embryonic kidney, and chicken embryo fibroblast can also appropriate for isolation and propagation of CMLV (Davies et al., 1975; Tantawi et al., 1974; Bhanuprakash et al., 2010a). CPE of CMLV in Vero cells may appear like rounding of cells, vacuolization of cytoplasm, formation of multinucleated giant cell with syncytia and cytolysis (Pfeffer et al., 1996; Marodam et al., 2006).
Serological Tests
Antibodies against poxvirus can be easily detected in animal’s serum than virus isolation (Marrennikova, 1975). CMLV and parapox antibodies never cross react and infections of camel orf and camelpox can be distinguished using serological methods. Several serological tests are available to identify camelpox which had been given in detail (Bhanuprakash et al., 2010b). Conventional serological tests like haemagglutination (HA), haemagglutination inhibition (HI), serum neutralization test (SNT), ELISA, Western blot analysis, complement fixation test (CFT) and fluorescent antibody tests (FAT) were utilized for detection of CMLV antibodies (Al Hendi et al., 1994; Marrenikova et al., 1974; Schgal, 1977; Davies et al., 1975; Bhanuprakash et al., 2010b; Tantawi et al., 1978; Al-Falluji et al., 1979; Azwai et al., 1996). SNT is the routinely used confirmatory test for OPV diagnosis in majority of laboratories (Boulther et al., 1971).
Molecular Diagnostic Tests
In recent times, PCR based on species specific C18L gene have been utilized to distinguish CMLV from buffalopox virus (BPXV) and other OPVs (Balamurugan et al., 2009). OPV genus specific PCR based on A-type inclusion protein (ATIP) gene of CMLV yields 881 bp specific amplicon (Meyer et al. 1994), while, PCR based on hemagglutinin (HA) - gene yields 1100 bp amplicon (Damaso et al., 2000). Later, the species- specific (CMLV) single PCR assays were employed to confirm CMLV. CMLV specific tumor necrosis factor binding protein receptor-II (TNFR-II) gene gave 270 bp amplicon (Lapa et al., 2002; Marodam et al., 2006). Further, PCR based on C18L gene and DNA pol gene also developed (Balamurugan et al., 2009) Diagnosis of OPV genome by real-time quantitative PCR was developed by Nitsche et al. (2004) which were more quick and reliable method for OPV and VARV diagnosis. Both SYBR Green based (Balamurugan et al., 2009) and TaqMan probe based (Venkatesan et al., 2012a) real time PCR developed for brisk and sensitive method of CMLV detection. The fluorescence resonance energy transfer (FRET) based real-time PCR assay also useful in accurate diagnosis of CMLV infection (Panning et al., 2004). Of late, Venkatesan et al. (2012b) developed a simple and rapid field application diagnostic tool, called loop-mediated isothermal amplification assay (LAMP) which targeted C18L gene and evaluated with samples from field. This novel approach is specific and highly sensitive, which can be performed in rural diagnostics laboratories in developing countries like India.
Prevention and Control Strategies
Prevention and control measures against sporadic cases of camelpox infection are very important in countries like India (Balamurugan et al., 2013). The restriction of animal movement, sanitary measures and isolation of the infected animals from healthier ones restrict the spread of the infection. Vaccination is the most economical and effective method to control camelpox. It is not necessary to vaccinate camels in all over the world. As an alternative, ring vaccination strategy can be employed during outbreak. It was more successful in last phase of the smallpox eradication campaign, in which rigorous and thorough monitoring and surveillance of disease was utilized for disease diagnosis followed by immunization of all animals in nearby associates and further sustained monitoring of disease to make sure no more diseases (Duraffour et al., 2011). Protective immunity in camelpox infection is both humoral and cell mediated (Elliot et al., 2008). Nevertheless, antibodies in circulation may not necessarily correlate with protective immune status of the animal (Wernery and Kaaden, 2002). Recovered animals become lifelong immune to re-infection. Live attenuated vaccines provide longer duration of protection for at least 6 years (Wernery and Zachariah, 1999), and inactivated viral vaccine offers protection for one year (Elliot et al., 2008).
Therapeutic Management
Therapeutic management for post exposure camelpox infection has not been reported in detail yet. Treatment approaches in severe cases were aimed in control of secondary bacterial infections either by parental or topical applications of broad spectrum antibiotics or antimicrobials and vitamins (Wernery and Kaaden, 2002). The alternative treatment could be use of antiviral agents to manage camelpox infections, particularly in young camels. HDP-cidofovir or CMX001 is the recently developed potential oral anti-poxviral drugs. Nevertheless, in few cases, some resistant camelpox viruses are very hard to treat with cidofovir, but virulence of these viruses may be attenuated in due course of treatment (Smee et al., 2002). Another novel compound named ST-246 which is a potent and effective against majority of OPVs including CMLV. The activity of the molecular compounds like CMX001, Cidofovir, and ST-246 have been tested and evaluated only in vitro and are of potent inhibitors of CMLV replication (Duraffour et al., 2010; 2011). Further, ST-246 and CMX001 can be administered orally which is more attractive and promising in veterinary field (Duraffour et al., 2007).
Vaccines
In earlier days, an ancient method of scarification with mixture of camelpox infected crusts and milk (lactotherapy) was practiced in Punjab region of India (Leese, 1909), past USSR and Arabian Bedouin to contain camelpox infections (Bhanuprakash et al., 2010a). The self-limiting nature of camelpox virus infection in humans reveals that it might be used as live virus vaccine against smallpox and old records point out that immunization of camelpox crust material was in use for that purpose in Iran, as long before cowpox method of immunization developed by Jenner (Tadjbahsh, 1994; Bray and Babiuk, 2011). Later in 1973, there was information on presence of camelpox virus vaccines from Russia, made out of VACV, not from CMLV due to antigenic similarity between CMLV and VACV (Buchnev et al., 1987). As result, it was more likely to vaccinate camels with vaccinia strain virus to prevent camelpox infections. Two outbreak of camelpox infection in Iran (Baxby et al., 1975) and Bahrain (Higgins et al., 1992) were effectively contained by vaccinating camels with the vaccinia-strain EA8 and vaccinia Elstree strain/Lister strain, respectively (Bhanuprakash et al., 2010a). Of late, several reports of inactivated camelpox vaccines with variable success have been reported from Saudi Arabia (Hafez et al., 1992), Morocco (EL-Harrak et al., 1991, 1998), and UAE (Wernery et al., 1997a; Kaaden et al., 1992; Wernery and Zachariah, 1999; Wernery and Kaaden, 1995; Wernery et al., 2000). The formalinized aluminium hydroxide adjuvanted camelpox virus vaccine (CMLV strain T8-1984) which gives protection from infection for one year is available in Morocco (Elliot et al., 2008). This vaccine is prepared and distributed by Biopharma® which was reported to be safe in young and adult camels, further it induces neutralizing antibodies against CMLV (El Harrak and Loutfi, 2000) and necessitates annual booster immunization.
At the same time, attenuated strains of viruses were utilized in UAE and Saudi Arabia for manufacturing attenuated live vaccine (Nguyen et al., 1996). The UAE research group developed the stable fetal dromedary skin cell line (Dubca) for isolation and characterization of CMLV (Klopries, 1993; Kaaden et al., 1992; Kaaden et al., 1995) which conferred protective immunity for maximum of 6 years following immunization in two animals. Even then, secondary booster immunization is suggested in young animals less than 6 months of age (Hafez et al., 1992). Of late, India has been able to produce a live Vero cell attenuated vaccine using an indigenous isolate (Bhanuprakash et al., Unpublished data). However, there is imperative to have a thermostability of these poxviral vaccines which has been studied in detail for the same camelpox vaccine (Prabhu et al., 2014), which may make possible their use in dry and hot regions of the country where outbreak of disease is common.
BUFFALOPOX
Buffalopox is a highly contagious acute viral disease of buffaloes, with 80% morbidity in affected herd population (Bhanuprakash et al., 2012). The first recorded incidence of buffalopox was reported in Lahore of undivided part of India in 1934 (Sharma, 1934) and was followed by frequent reports of outbreaks from various states of India (Sehgal et al., 1977). Disease also reported from Nepal, Bangladesh, Pakistan and Egypt (Singh et al., 2007a). In buffaloes the disease outbreaks are may be allied with elevated morbidity rate and loss of production (Chandra et al., 1987; Singh et al., 2006a, 2006b, 2006c, 2007a). The severity of the infection ranges from localized mild form to the infrequent severe generalized form. The infection poses economic impact due to reduced milk yield resultant from mastitis in milch animal and reduced working capacity in draft animals (Bhanuprakash et al., 2010c). Although buffaloes are commonly infected, cattle and human beings are infrequently get infection. Transmission potential of BPXV includes cattle, buffaloes and humans imply the potential re-emergence of the infection in Indian subcontinent as like VLV outbreaks observed in other countries in recent years (Singh et al., 2012).
Disease Classification
The Joint Expert Committee on Zoonosis confirmed buffalopox is one of the potential zoonotic diseases and transmission mode of buffalopox to human beings are alike that of cowpox infections (FAO/WHO, 1967; Chandra et al., 1987). In human, gross lesions may appear in animal attendants and milk-men who are in close proximity with affected buffaloes.
Geographical Distribution
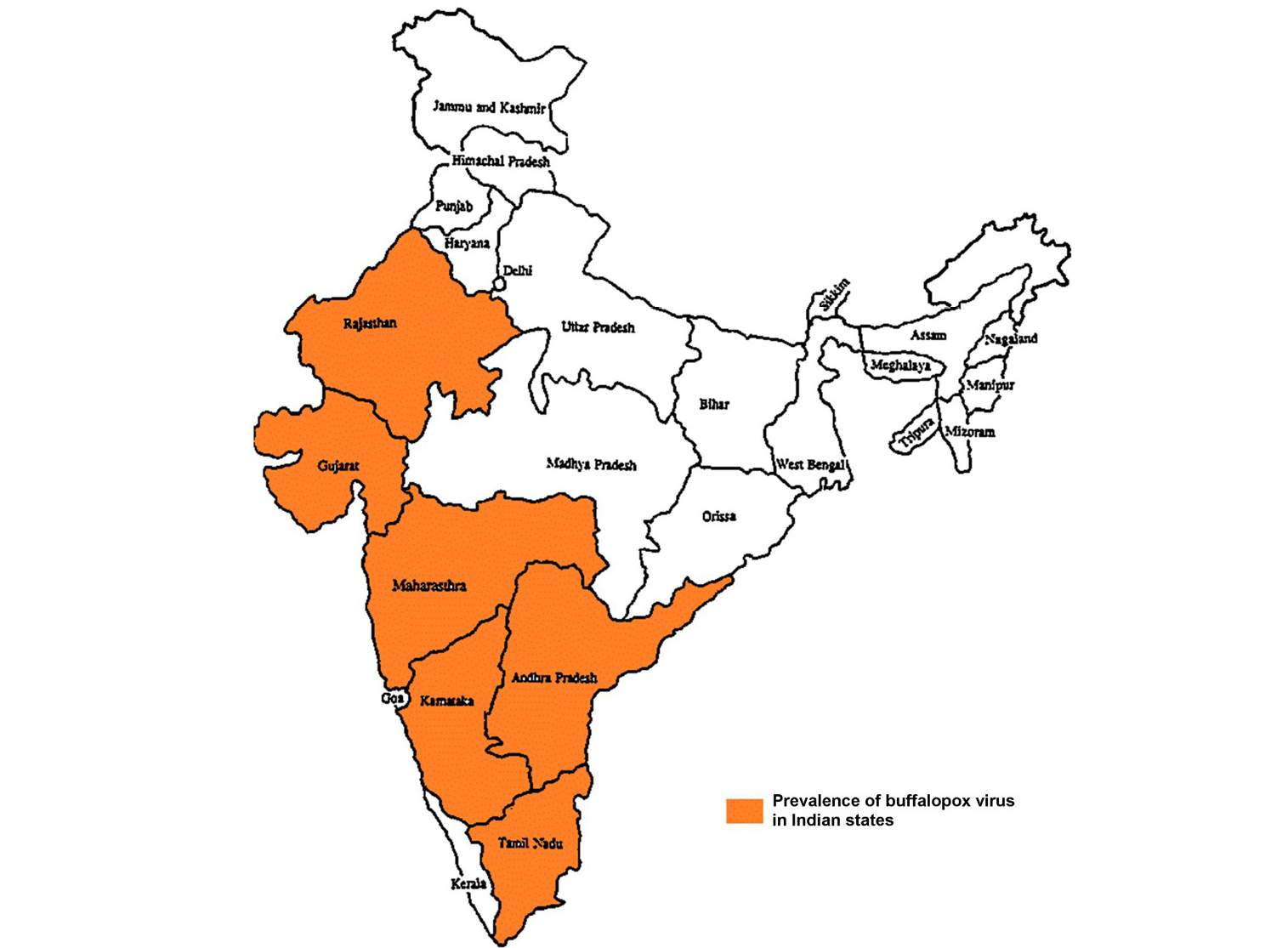
Buffalopox is endemic with increasing trend in India (Prabhu et al., 2012) and outbreaks are reported from Maharashtra, Gujarat, Rajasthan, Andhra Pradesh, Tamilnadu and Karnataka states in India (Figure 3) (Venkatesan et al., 2010b; Bhanuprakash et al., 2010c; Chandranaik et al., 2011; Gurav et al., 2011). Apart from Egypt, India, it is also been reported from several other countries namely Italy, Russia, Pakistan, Indonesia, Bangladesh and Nepal (Singh et al., 2012). In Pakistan, buffalopox caused a nosocomial infection in human beings (Zafar et al., 2007; Milton et al., 2015).
Etiological Agent
The etiological agent of buffalopox infection in buffalo is bufflopox virus (BPXV). It is grouped in the subfamily of Chordopoxvirinae in the genus Orthopoxvirus (OPV) in the family Poxviridae (Van Regenmortel et al., 2000). The BP4 (Hisar) strain is considered as reference virus strain in India (Singh and Singh, 1967; Baxby and Hill, 1969). BPXV is close to the clade of VACV. In addition, studies on analyses of envelope (Singh et al., 2006b) and B5R (immunogenic proteins) genes (Singh et al., 2007b) of BPXV exposed a close association between BPXV and VACVs. Further, nucleotide and deduced amino acid sequences showed high identity of sequence with VACV (99%) and C18L gene showed that BPXV isolates clustered into a different group which is distinct from VACV (Singh et al., 2008 and 2012).
Physicochemical Properties of BPXV
BPXV is least sensitive to ether and sensitive to bile salts, chloroform, extreme ranges of pH and heat (Lal and Singh, 1977). Heat inactivation of BP4 strain virus to 56oC for 90 minutes resulted in complete loss of infectivity (Chandra et al., 1987), whereas BPXV isolated from 1980 outbreak in Hisar, Haryana, India (strain BPH-80) showed loss of infectivity at 56oC in 60 min (Kumar et al., 1987). Heat inactivation rate is higher in the presence of bivalent Mg++ cations. Though sensitive to heat, the BPH-80 strain of BPXV can produce pock lesions on CAM of embryonated chicken eggs incubated at 40.5±0.5oC, in contrast BP4 strain of BPXV failed to produce such lesions. But, all these strains showed pock lesions at 38±0.5oC incubation (Kumar et al., 1987; Singh et al., 2007a).
Host Range
Buffalopx is mainly a disease of domestic buffaloes (Bubalus bubalis) (Venkatesan et al., 2010b), and occasionally causes limited lesions in milch cows and occasional oro-pharyngeal lesions in human beings due to consumption of unpasteurized milk (Damaso et al., 2000; Dumbell et al., 1993). Buffaloes of all age groups and both sexes are affected, but the disease is severe in young and old animals (Bhanuprakash et al., 2010c). Experimentally, the buffalopox can be transmitted to buffaloes, cows (high dose of virus), guinea pigs, rabbits, suckling mice of Swiss white and Balb/C strain and chickens. But sheep, goat, fowl and adult mice of Swiss white and Balb/C strains are not suitable for experimental BPXV infections (Kumar et al., 1987).
Disease Transmission
The buffalopox transmission in animals is natural. In humans, attendants and milkmen who are in close proximity with affected buffaloes are usually getting affected (Singh et al., 2006a). Though the vector transmission is not been proved, biting flies like Musca vicina, Musca crossirostris and Lyperosia exigua have been reported to aggravate sores in animals. Therefore, it has been believed that flies may be implicated in transmission of BPXV mechanically (Muraleedharan et al., 1989). The influence of environment and predisposing factors in the occurrence of buffalopox infection has not been extensively studied and established. An increased transmission of BPXV in variable species, cows, buffaloes and human beings, suggests the re-emergence of buffalopox zoonoses (Bhanuprakash et al., 2010c; Venkatesan et al., 2010b). The outbreaks observed in different remote location of India may represent abundant of natural BPXV reservoir wild animals especially rodents. Hence, large scale study on the prevalence OPV in wild animals in India is of prime important (Shchelkunov, 2013).
Prevalence of Disease in Animals and Human Beings
The occurrences of buffalopox in buffaloes are allied with variable rate of morbidity, mortality and case fatalities. Mortality and case fatality rates are usually low. Human infection followed by outbreak in buffaloes has been reported since its first recorded incidence. Haemagglutinin (HA) and ‘A type’ inclusion (ATI) gene sequences pox virus isolates for cattle revealed that BPVXs circulates in cows too (Yadav et al., 2010) along with buffaloes and humans
Table 1: Recent zoonotic outbreaks of buffaloxpox and camelpox infections at different places of India
|
Area-Village/ State |
Year |
Number of Human cases |
Identification/ confirmation test |
References |
|
Camelpox zoonosis |
||||
|
Rajasthan, India |
2009 |
3 |
||
|
Buffalopox zoonosis |
||||
|
Aurangabad, Maharastra |
2003 |
- |
CIE, SNT, PCR and Virus isolation |
|
|
TamilNadu |
2005-06 |
- |
- |
Unpublished data; Singh et al. (2012) |
|
Kolhapur- Pune, Maharashtra |
2009 |
125 |
CIE, SNT, PCR, TaqMan PCR |
|
|
Thotapalligudur- Nellore, AndraPradesh |
2006-08 |
7 |
CIE, SNT |
|
|
Aurangabad (Kathiawad region) |
2006-08 |
5 |
CIE, PCR, Real time PCR, Virus isolation |
|
|
Aundh – Pune, Maharashtra |
2006-08 |
4 |
SNT |
|
|
Ratadia - Sardar Krishinagar, Gujarat |
2006-08 |
9 |
CIE |
|
|
Bavar - Sardar Krishinagar, Gujarat |
2006-08 |
11 |
CIE |
|
|
Hisar |
2014 |
1 –Laboratory acquired |
PCR |
|
(Singh et al., 2006a, 2007a; Kolhapure et al., 1997).
Clinical Manifestation in Animals
The incubation period in animals is 2–4 days (Ghosh et al., 1977). The disease occurs both in mild and severe forms. In localized less severe form lesions are confined to the inguinal region, udder and teats (Sharma, 1934; Singh and Singh, 1967), above parotid gland, inner aspect and base of eyes and ear (Bhatia, 1936; Wariyar, 1937; Mallick and Dwivedi, 1982; Mallick, 1988); Secondary bacterial otitis may appear. Whereas in severe form (Chandra et al., 1987; Ramakrishna and Ananthapadmanabham, 1957) lesions are normally generalized and seen on the teats, udder, thighs and hindquarters of infected animals. In severe infections in milking animals may causes mastitis, with everlasting milk yield reduction in (Singh et al., 2006a). Further it leads to reduced working capacity of draft animals (Singh et al., 2007a; Venkatesan et al., 2010b).
Zoonotic Significance
Buffalopox is an important zoonotic disease. Recurrent epidemics and transmission of BPXV in cattle, buffalo and human beings make a very vital public health apprehension after the termination of vaccination against smallpox worldwide (Raut et al., 1997; Kolhapure et al., 1997; Singh et al., 2006a, 2007a). Outbreaks of diseases were reported at some point of smallpox immunization in Egypt, and Indonesia and later from India (Kolhapure et al., 1997; Nedunchelliyan et al., 1992; Singh et al., 2006a), Pakistan (Qureshi, Personal communication) and Nepal (Venkatesan et al., 2010b). The report on recent zoonotic outbreaks is given in Table 1. Human including individuals immunized with smallpox vaccines also contract infection due to intimate association with infected animals, further, severity and extent of disease is much higher in non-vaccinated persons (Singh et al., 2007a). Disease mainly spread by milking of infected animals. Since early, manifestation of diseases in humans was observed in persons associated with pox-infected buffaloes, animal attendants and milkers. (Wariyar, 1937; Mehrotra et al., 1981; Ghosh et al., 1977; Kumar et al., 1987; Raut et al., 1997; Kolhapure et al., 1997; Singh et al., 2006a). The incubation period in humans ranges from 3–19 days after exposure (Ghosh et al., 1977). Both the genders and all age groups of human beings contract the disease. The clinical signs in human include fever (39.0oC – 40.5oC) for 2-3 days, subsequently skin eruptions may appear. The lesions appear as small, round nodule with umbilication with an erythematous base. The lesions sometimes contain pustular material with cheesy consistency. The size may increase from1-2 mm to 1 cm in diameter, and severity persists for 7-8 days (Zafar et al., 2007). BPXV lesions are similar to cowpox vesicular lesions, but less painful, in some cases may appear like VACV lesions. The gross lesions observed in face, forehead, hands, buttocks and legs with lymphadenopathy of dependent parts Transmissions between humans are not been documented (Bhanuprakash et al., 2010c; Gurav et al., 2011). However, after detailed analysis Kolhapure and his colleagues (1997) reported that the presence of serum antibodies in more than 70% of in-contact individuals and 17% of persons with no history of association with infected humans or buffaloes is a public health alarm and finding of antibodies in these persons may be the signal of subclinical infection which may act as reservoir common human population to zoonotic BPXV. Furthermore, these viruses may obtain virulence after repeated passage in human beings which is obvious when comparing isolation of BPXV strains in recent potbreak and the reference strain (BP4) isolated about 40 years ago (Kolhapure et al., 1997; Singh et al., 2007a).
Diagnostic Procedures
Clinical diagnosis can be made on the basis of clinical picture of typical pox lesions on udder, teats or affected body parts. But, it must be differentiated from other diseases affecting udder like cowpox, pseudo-cowpox and bovine herpesvirus mammilitis. Laboratory investigations are, therefore, essential for establishing a confirmatory diagnosis.
Virus Isolation
As like CMLV, for isolation of BPXV, embryonated chicken eggs, primary cell culture and cell lines are used. 10% scab suspension prepared in phosphate buffer or normal saline is used to inoculate either chorio-allantoic membrane of chickens or specific hosts like buffalo or cattle. The virus produces large (0.8-1.5mm diameter) raised or flat and white or grey pocks without hemorrhage or central necrosis on chorioallantoic membrane (CAM) of chick embryo after 48-72 hrs infection and later the death of embryo (60-70% mortality). BPXV has been propagated in rabbit kidney (RK-13) cells, chicken embryo fibroblast cells, HeLa cells, pup kidney cell culture, Vero, BHK21 and also in BHK21 suspension cell cultures. BHK21 cells are more sensitive for primary isolation of BPXV from clinical samples. The virus produces plaques of about 0.8 mm size diameter on RK-13 cells, 1-2 mm diameter on chicken embryo fibroblasts and 0.8-1.2 mm diameter on Vero cells. The cytopathic effect includes clumping and granularity in cytoplasm, rounding, cluster formation and syncitia. BPXV exhibits characteristic ‘rosette’ or ‘sun flower’ like haemadsorption with 0.4% fowl erythrocytes (Sehgal et al., 1977).
Serological Tests
Serological assays for detection of buffalopox infections includes agar gel immunodiffusion test (AGID), serum neutralization test (SNT), counter-immunoelectrophoresis (CIE), immunoperoxidase test (IPT) and ELISA (Bhanuprakash et al., 2010c). In gel diffusion tests cross reactivity of BPXV was observed with sheeppox virus (SPPV), goatpox virus (GTPV) and fowlpox virus (FPV) (Mathew, 1975). Further, BPXV antiserum may also cross-reacts with FPV in the HI test, but rabbit anti-VACV serum may not react with FPV in same test (Mathew, 1975). Cross-precipitation and cross-neutralization tests revealed the subsistence of a close association of BPXV with VACV (Lal and Singh, 1973; Kataria and Singh, 1970; Singh and Singh, 1967; Baxby and Hill, 1971). Nevertheless, no cross reacting precipitation of BPXV was observed in swinepox virus (SWPV) in agar gel precipitation test (Lal and Singh, 1973; Baxby and Hill, 1971; Kataria and Singh, 1970; Singh et al., 2007a).
Molecular Diagnostic Tests
Several limitations are there in identification and differentiation of OPV’s by conventional serological and biological methods. Analysis of viral polypeptides with sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) facilitated differentiation of genus and species but closely associated members of same genus cannot be differentiated. However, restriction enzyme-DNA (RE–DNA) profiling can be utilized for identification of poxvirus but it necessitates propagation of virus and isolation of viral genomic DNA, which requires more time (Singh et al., 2007a). PCR based on HA genes have been used for diagnosis and differentiation of OPVs. Primers viz. CoPV-3 and CoPV-4 has been applied for diagnosis and differentiation of OPVs including CMLV, CPXV, MPXV, raccoonpox virus (RCNV), VACV and BPXV. These primers amplify 2.8, 3.7 and 3.2 kbp fragments in CMLV, CPXV and BPXV, respectively (Ropp et al., 1995). Inclusion gene is contained in two fragments of HindIII digest of BPXV genomic DNA. Primer pair of ATI-up and ATI-low amplify the fragment of the inclusion gene which is also utilized for discrimination of OPVs. The amplified product size in BPXV is 1587 bp whereas1603, 1673 and 880bp in VACV-WR strain, CPXV-Brighton strain and CMLV CP-1 strain, respectively (Singh et al., 2007a). Further, DNA of CMLV and BPXV can be characterized and differentiated by using DNA amplification fingerprinting (DAF) by RAPD-PCR. At last, TaqMan probe-based quantitative real-time PCR (qRT-PCR) based on Ankyrin repeat protein (C18L) gene and duplex PCR based on DNA polymerase (DNA Pol) and C18L are used for discrimination of BPXV from other OPVs (Singh et al., 2008).
Prevention and Control Strategies
The control measures followed to manage camelpox can be applied for buffalopox too. Disease control measure is very difficult to imply in certain countries, in which the disease is endemic in nature and the restriction of animal movement is very difficult. In addition, the export of contaminated buffalo meat is major concern. Cultural issues, social beliefs and economic contemplation put off India from having a policy of buffalo/cattle slaughter (Singh et al., 2007a). In the absence of appropriate immunopropylactic measures, movement control of animals, implementation of hygienic measures like isolation of the diseased animals is the effective method to control the spread of infection.
Therapeutic Management
Certain drugs effective against OPV either in vitro and/or in vivo are available viz.cidofovir, ST-246, STI-271, CMX001, cell penetrating peptides, imiquimod, gefitinib, nigericin, and herbal extracts (Kroon et al., 2011; Bhanuprakash et al., 2012). Likewise, yet another remedial approach is the use of siRNA can also be employed. Consequently, recent studies on VACV targeting D5R, E3L, B1R, and G7L genes in vitro have been developed. These strategies may be useful in treating buffalopox also (Kroon et al., 2011; Bhanuprakash et al., 2007).
Vaccines
Despite of zoonotic nature buffalopox, literature regarding immunoprophylaxis is scarce. Very few vaccines have been developed against BPXV infection, but none of the vaccine is available for commercial use. BPXV vaccines produced by β-propiolactone and formalin inactivated, saponin and aluminium hydroxide gel-adjuvanted vaccine were ineffective when challenged in rabbits (Mohanty et al., 1989a). Later, BPXV-BP4 strain adapted in chicken embryo fibroblast and Vero cells were reported to be effective in buffaloes and rabbits (Mohanty et al., 1989b) and rabbits, respectively (Dogra and Sharma, 1981). Further vaccine produced using field strain of buffalopox (BPXV-Vij96) virus caused buffalopox outbreak in Andhra Pradesh (India), attenuated in Vero cells has been developed at Indian Veterinary Research Institute, Mukteswar, Uttarakhand (Singh et al., unpublished data). Preliminary safety, efficiacy and immunogenicity studies of both BPXV-BP4 and VIJ96 attenuated virus vaccine candidates were studied in buffalo claves by experimental challenge revealed both the vaccines were safe and potent. Both vaccines were potent and efficient and as confirmed by seroconversion with raise in antibody titer and protection following challenge in buffalo calves. The warrant protective immune response, prepared vaccine was given intradermally at ventral aspect of the tail at the dose rate of 103 tissue culture infective dose (TCID50) (Bhanuprakash et al., 2012).
Conclusion
Cessation of immunization against smallpox infection due to its eradication since 1980’s resulted in vulnerable naïve human population against diverse OPVs throughout the world. This altered nature of virus infections in humans were may be due to VACV vaccine strain adaptation in animals by continuous passage or intimate contact of vaccinated human beings with pet animals. Although it was believed as host specific and infects only camels, the recent report of camelpox advocates a possible public health impact of the disease and necessitates further investigation. If the situation continues with greater number of outbreaks than early, it may be the next target for us after the eradication of smallpox infection and poliomyelitis in humans and rinderpest in animals. Considering the increased incidence of disease, molecular epidemiology of virus, disease diagnosis and control measures are very important in reducing the transmission of CMLV in camels and humans in terms of public health significance. Control of emerging buffalopox infection in animals will be vital which may be disease of zoonotic significance. The condition is much deteriorated in present situations due to increased outbreak of buffalopox infections. It seems that the BPXV isolated from human beings in India increasingly adapted to human beings because of intimate association of humans with infected animals. Since chemotherapy against buffalopox is ineffective, further, use of antibiotics/antimicrobials to treat secondary bacterial infections in infected animals may predispose human health vulnerability due to chances of residual antibiotics residues in meat and milk.
Meat form BPXV infected buffalo has several related implications at international level. This signify the requirement of comprehensive systematic investigations on molecular epidemiology, subsistence of reservoir host, transmission and molecular association of BPXV and CMLV in association with related viruses, which may help in implementation of preventive and control measures. Eradication of diseases at global level conventionally been related with actions on a global or international scale, as like present movement to eradicate poliomyelitis in humans. However, such kind of huge efforts may not be necessary for diseases like buffalopox and camelpox, due to the fact that outbreaks are confined to certain regions could be controlled.
ACKNOWLEDGMENT
The authors are thankful to the Pox Laboratory, Division of Virology, Mukteswar (Regional Station), ICAR-IVRI for the support. The authors also thankful to Dr. V. Bhanuprakash and Dr. V. Gnanavel for their kind support during camelpox and buffalopox research.
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHORS CONTRIBUTION
Manimuthu Prabhu, Revanaiah Yogisharadhya and Raj Kumar Singh provided the main concept and designed the study. Chinnathambi Suresh and Gopal Sathish managed acquisition of data while Manimuthu Prabhu and Selvaraj Pavulraj analysed and drafted the manuscript.
REFERENCES