Advances in Animal and Veterinary Sciences
Research Article
The Protective Effect of Pometone (Pomegranate Seed Oil) on Oxidants /Antioxidant Status in Methionine Overload Rabbits (Part III)
Baraa Najim Al-Okaily*, Lekaa Najim Abdulla, Khalisa Khadim Khudair
Department of Physiology and Pharmacology, College of Veterinary Medicine, University of Baghdad-Iraq.
Abstract | This study was aimed to investigate the role of pomegranate seed oil (PSO) as antioxidant in alleviating the deleterious effects of methionine overload in some biochemical aspects of adult female rabbits. Thirty two female rabbits were randomly assigned into four equal groups and given orally the following treatment for 42 days: the first groups was drenched corn oil, serving as control group (C), the second group (T1) was intubated with methionine 100mg/kg. B.W, the third groups (T2) was intubated with methionine 100mg/kg. B.W and pomegranate seed oil (PSO) 30 mg /Kg. B.W., while animals in the group T3 were intubated with pomegranate seed oil 30 mg/Kg. B.W. Fasting blood samples were collected at 0, 21 and 42 days of experiment to assess: serum total cholesterol(TC), malondialdehyde (MDA), peroxynitrite radicals, reduced glutathione (GSH) concentrations and lactate dehydrogenase activity( LDH). At the end of the current study group T1 showed a significant increase in TC, MDA, peroxynitrite radicals concentrations and LDH activity with significant reduction in GSH concentration. Besides the results showed a significant decrease in serum TC and peroxynitrite radical concentrations with significant elevation in GSH concentration in group T3 as compared with the control. It can be concluded that intubation of PSO correct the oxidative stress in methionine overload rats.
Keywords | Pomegranate seed oil, Methionine, Oxidative stress, Antioxidants
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 06, 2015; Revised | May 25, 2015; Accepted | May 27, 2015; Published | June 07, 2015
*Correspondence | Baraa Najim Al-Okaily, University of Baghdad, Iraq; Email: baraanajim@yahoo.com
Citation | Al-Okaily BN, Abdulla LN, Khudair KK (2015). The protective effect of pometone (pomegranate seed oil) on oxidants /antioxidant status in methionine overload rabbits (part III). Adv. Anim. Vet. Sci. 3(7): 377-383.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.7.377.383
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Al-Okaily et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Methionine is an essential amino acid found in both animal and plant proteins. Methionine is a protective factor against various types of liver damage, but excessive dietary methionine is hepatotoxic (Sukhanov et al., 2013). When the metabolic pathways of methionine are interrupted due to malnutrition or gene mutation, homocysteinemia (Hcy) level will be elevated (Jakubowski, 2006). New suggestion revealed that hyperhomocysteinemia (HHcy) play an important role in different physiological disorders including, lipid peroxidation (Bouzouf et al., 2005), oxidative stress (Delvin et al., 2007; Vanzin et al., 2011), cardiovascular commitment (Refsum et al., 1998; Tounyz and Schiffrin, 2008), Alzheimer disease (Hooshmand et al., 2013) and depression (Nabi et al., 2013). Moreover, Hcy has been shown to affect lipid metabolism, which in turn contributes to the development of atherosclerosis (Zhang et al., 2012).
Punicagranatum L. is used from ancient times and reports of its therapeutic qualities has echoed throughout the ages. The pomegranate fruit have many functional and medicinal effects. Pomegranate flowers attenuate aging-mediated undesirable skin abnormalities (Wang et al., 2012), besides possessing potent antioxidant and hepatoprotective activity (Kaur et al., 2006) hypoglycemic (Huang et al., 2005) properties and cardioprotective effect (Mohan et al., 2010; Al-Okaily and Abdullah, 2014). Whereas, the pomegranate peel has been shown to have anti-inflammatory (Bachoual et al., 2011), anti-mutagenic (Zahin et al., 2010) and antifungal activity (Endo et al., 2010). Pomegranate seeds are by-products of pomegranate juice (PJ) and they can be useful for food applications as a functional agent (Tehranifar et al., 2010; Mohagheghi et al., 2011). Recent studies found that pomegranate seed have been regarded as a good source of nutrients and antioxidants (Aviram 2000). It has been suggested that dietary supplementation with pomegranate seeds may reduce weight gain and risk of type 2 diabetes (Strohacker and Kueht., 2009), prevention of cancer (Lansky and Newman., 2007) and also reduce menopausal symptoms (Auerbach et al., 2012). The health effects of pomegranate seeds may be related to the presence of a variety of biologically active compounds (Viuda-Martoset al., 2010), particularly polyphenols (Balasundram et al., 2006). Besides, significant levels of phenolic content were detected in pomegranate seeds (Pande et al., 2009; Elfalleh et al., 2011).Therefore, this study was aimed to evaluate the protective role of pometon (PSO) against deleterious effect induced by methionine overload on oxidant/antioxidant status in adult rabbits.
MATERIAS AND METHODS
Thirty two 6-8 months female local rabbit weighing 1250-2000gm were used in this study. Animals were housed in cages in conditioned room (22-25°C) in the animal house of College of Veterinary Medicine -University of Baghdad, the experiment commenced from November 2012 till February 2013. Animals had free access to water and standard pellet diet consisting according to Lab Diet Rodent Diet 5001 (PMI Nutrition International, St. Louis, MO, USA). Rabbits were randomly divided into four equal groups and treated for 42 days. Group(C): administered ordinary corn oil orally, serving as control, group T1 were administered methionine 100 mg/kg B.W (Segma), group T2 treated with same dose of methionine plus pmetone (PSO) 30mg/kg BW (vitane pharma Gmph-Germany) and group T3 were treated with PSO alone at same dose in T2. Fasting blood samples were collected at 0, 21, 42, days of the experiment by cardiac puncture technique (cardiocentesis) by using vacuum tube with gel colt activator. Isolated serum was collected and kept at -20°C until analysis for measuring the concentration of: serum total cholesterol (TC) using enzymatic kit (Linear Chemicals S.L,Spain), malondialdehyde (MDA) was determined according to (Guidet and Shah,1989), peroxynitrite radical (Van Uffelen et al., 1998) and reduced glutathione (GSH) was determined by method as described by Burtis and Ashood (1999). Besides, serum lactate dehydrogenase (LDH) activity according to (King, 1965). The concentration of serum GSH micromole/litre was obtained from the standard glutathione curve (Figure 1).
Statistical Analysis
The results are expressed as mean ±SE. Analysis of data was performed on the basis of Two-Way Analysis of Variance (ANOVA) using a significant level of (P<0.05). Specific group differences were determined using Least Significant Differences (LSD) (Snedecor and Cochran, 1973).
Results and Discussion
Table 1 showed a general trend for the TC values to increase significantly (P<0.05) after 21and 42days in group T1 compared with groups C, T2 and T3.On the other hand, serum TC concentration dropped significantly (P<0.05) at day 42 of the experiment in groups T2 compared to group T1. Besides, PSO intubation to normal rabbits in group T1 caused significant decrease in this parameter along the experimental period comparing to others group.
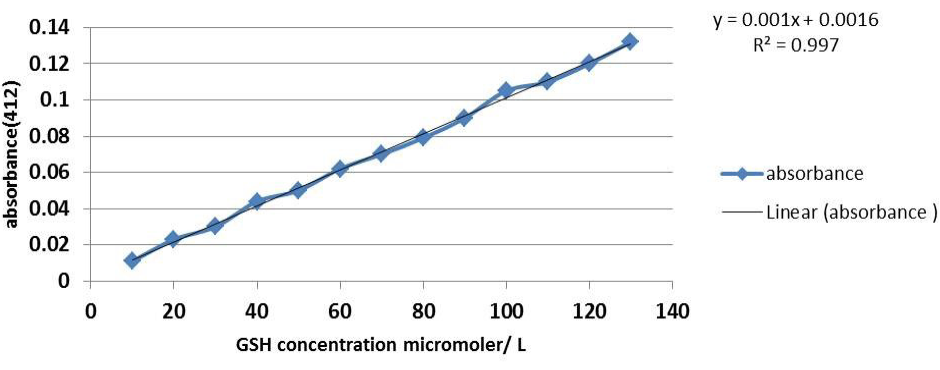
Figure 1: Standard curve of GSH
Table 1: Effect of pometone on serum total cholesterol (mg/dl) in methionine overload female rabbits
|
Groups |
Day |
|||
|
T3(methionine +PSO) |
T2 (PSO30 mg/kg BW) |
T1 (methionine 100 mg/kg BW) |
Control |
|
|
93.44±0.6 A a |
94.34±0.9 A c |
93.92±1.1 A c |
93.97±0.7 A a |
0 |
|
84.35±0.9 D b |
110.84±0.4 B b |
116.87±0.5 A b |
94.51±0.6 C a |
21 |
|
66.21±0.5 D c |
127.91±0.5 B a |
183.50±0.6 A a |
95.48±0.8 C a |
42 |
Values are mean ± SE, n=8; Small letters denote within group difference p<0.05. Capital letters denote between groups difference p<0.05.
The results showed that oral administration of rabbits to methionine overload for six weeks (group T1) caused pronounced increase in serum cholesterol concentration comparing to control and other treated groups (T2, T3).These results are in agreement with other studies (Delvin et al., 2007; Ventura et al., 2000). HHcy plays an important role in cholesterol biosynthesis by inducing transcription as well as translation of 3-hydroxy 3-methyglutaryl co-enzyme reductase. It also increase cholesterol synthesis and intestinal absorption which suppressed cholesterol excretion (Ventura et al., 2000). Besides elevated Hcy may impaired nitric oxide (NO) through depression in plasma cysteine (Leoccini et al., 2003 and Tanriverdi et al., 2007), the most important amino acid for cholesterol excretion, and salt formation (Finkleston and Holbrook, 2000). Accordingly, Hcy after methionine overload could be attributed to hyperlipidemia observed in this study. Hcy can potentially induced endoplasmic reticulum (ER) stress, activates and induce expression of sterol regulatory binding proteins-1, an ER membrane bound transcription factors of vascular endothelium and human hepatocytes, leading to elevate in biosynthesis and uptake of cholesterol , triglycerides with accumulation of cholesterol (Werstuk et al., 2001; Woo et al., 2005). The high capability of PJ to scavenge free radicals, inhibit LDL oxidation in vivo and in vitro (Gil et al., 2000; Aviram et al., 2000 and 2002) and reduction in oxidative stress (Salonen et al., 2000 and 2003)may be due to high content of polyphenols which responsible for its hypolipidemic effect.
Table 2: Effect of pometone on serum malondialdehyde concentration in methionine overload female rabbits
|
Groups |
Day |
|||
|
T3 (methionine +PSO) |
T2 (PSO30mg/ kg. BW) |
T1 (methionine 100 mg/kg BW) |
Control |
|
|
0.26±0.004 A c |
0.25±0.003 A c |
0.25±0.003 A c |
0.25±0.004 A a |
0 |
|
0.23±0.004 C b |
0.30±0.005 B b |
0.36±0.007 A b |
0.26±0.005 D a |
21 |
|
0.24±0.003 C a |
0.38±0.004 B a |
0.45±0.007 A a |
0.25±0.005 C a |
42 |
Values are mean ± SE, n=8; Small letters denote within group difference p<0.05. Capital letters denote between groups difference p<0.05.
Statistical analysis of the results of MDA concentration at day 21 and 42 showed that intubation of animals with methionine (group T1) caused significant (p<0.05) increase compared to control, T1 and T3 (Table 2). Whereas, treatment of rabbits with methionine plus PSO (group T2) caused a significant (p<0.05) decrease in the serum MDA concentration at the same periods of treatment compared to group T1. PSO cause lowering in serum MDA concentration in group T3 and the results near that of control. The result showed that intubation of rabbits with methionine (group T1) caused a significant (p<0.05) increase in serum peroxynitrite concentration at two treated periods compared to control, T2, and T3 groups. Results have also clarified that treatment of rabbits with PSO (group T3) showed significant (p<0.05) decrease in the serum peroxynitrite concentration at 21 and 42 days of experiment compared to T1 group. Highest significant (p<0.05) reduction in serum peroxynitrite concentration were observed after pomegranate seed oil intubation to normal animals in group T3 comparing to T1 and T2 treated groups (Table 3).
A significant (p<0.05) decrease in serum GSH concentration were detected after 42 days of experiment in groups T1 and T2 comparing to control and T3 treated groups (Table 4). The results also showed that oral intubation of PSO to normal animals caused significant (p<0.05) increase in serum GSH concentration of treated rabbits (group T3) comparing to other treated.
Table 3: Effect of pometone on serum peroxynitrite radical concentration in methionine overload female rabbits
|
Groups |
Day |
|||
|
T3 (methionine+PSO) |
T2 (PSO30 mg / kg. BW) |
T1 (methionie 100 mg/kg. B.W) |
Control |
|
|
33.51±0.5 A a |
34.72±0.4 A c |
33.51±0.3 A c |
33.57±0.7 A a |
0 |
|
31.68±0.4 C b |
40.03±0.3 Bb |
42.43±0.4 A b |
32.08±0.4 Ca |
21 |
|
32.68±0.5 C b |
45.03±1.03 B a |
63.25±1.01 A a |
33.50±0.3 C a |
42 |
Values are mean ± SE, n=8; Small letters denote within group difference p<0.05. Capital letters denote between groups difference p<0.05.
Table 4: Effect of pomegranate seed oil on serum reduced glutathione concentration in methionine overload female rabbits
|
Groups |
Day |
|||
|
T3 (methionine+ PSO) |
T2 (PSO30 mg / kg. BW) |
T1 (methionine 100 mg / kg. BW) |
Control |
|
|
0.05±0.0010 A c |
0.06±0.0007 A a |
0.06±0.0007 A a |
0.06±0.0008 A a |
0 |
|
0.13±0.0006 A b |
0.05±0.0006 D b |
0.05±0.0010 C b |
0.06±0.0007 B a |
21 |
|
0.14±0.0010 A a |
0.05±0.0004 C b |
0.05±0.0005 D b |
0.06±0.0006 B a |
42 |
Values are mean ± SE, n=8; Small letters denote within group difference p<0.05. Capital letters denote between groups difference p<0.05.
The overall results of the present study demonstrated that treatment of rabbits with methionine overload induced an elevation in oxidative stress parameters, evidenced by increased serum MDA and peroxynitrate and decreased reduced GSH concentrations. Biological oxidative stress is defined by an imbalance between antioxidant and free radicals, and this phenomenon is associated with HHcy (methionine overload) (Petrak et al., 2007; Tounyz and Schifrin, 2008). It has well known that the free radicals initiate the peroxidation of membrane polyunsaturated fatty acids which results in the generation and excess production of reactive ROS and finally cell necrosis (Melin et al., 2000; Manna et al., 2011). The subsequent increase in serum MDA concentration in the current study may be attributed to high sensitivity of rabbits to free radicals production (by HHcy) induced by methionine over load. This elevation in generation of lipid peroxidation (MDA level) was postulated to cause a gradual cell injury by free radicals liberating lipoxygenase enzymes which oxidized unsaturated membrane fatty acids and subsequent production of MDA, overwhelming endogenous scavenging system including GSH resulting in oxidative stress (Stark, 2005; Handy and Zhang, 2006) .
It has been postulated that peroxynitrite mediated one-electron oxidation of biomolecules may be an important event in its cytotoxic mechanism (Augusto et al., 1994). The generation of peroxynitrate over long periods of time will result in substantial oxidation of potential destruction of host cellular constituents, leading to the dysfunction of critical cellular processes, disruption of cell signalling pathway, and the induction of cell death through both apoptosis and necrosis (Pacher et al., 2007).
A significant decrease in serum GSH concentration in methionine treated group in the present study is correlated with (Ventura et al., 2000; Huang et al., 2001; Hayden and Tyage, 2004). Many authors demonstrated that mild HHcy is much more common and is associated with postmethionine loading in water (Labinjoh et al., 2001; Bagi et al., 2003) or in diet (Apeland et al., 2003; Zhang et al., 2004). The predicted HHcy after methionine overload may decrease the ability of the cells to detoxify H2O2 and other lipid peroxide (Tounyz and Schifrin, 2008) and it might be indirectly produce oxidative reduction in the activity of intracellular antioxidant enzyme.
The present study showed that PSO supplementation during treatment of methionine overload exhibited an increase in GSH concentration and reduce MDA and peroxynitrate concentration, compared to methionine–treated group (T1). Phenolic compound (Kaur et al., 2006) present in juice , including punicalagin, ellagic acid (Cuzzocree et al., 2001; Chen et al., 2012) and anthocyanins (Seeram et al., 2005) are known for their properties in scavenging free radicals and inhibiting lipid oxidation and elevation antioxidants capacity of the body. The mechanisms of antioxidant effects of PJ may include: 1) suppressing reactive oxygen species formation either by inhibition of enzymes or chelating trace elements involved in free radical production, 2) scavenging reactive oxygen species, 3) up regulating or protecting antioxidant defenses (Grassi et al., 2009a; Grassi et al., 2009b; Ferri and Grassi, 2010) and 4) increasing resistance to oxidative stress (Li et al., 2006; Bachoual et al., 2011).
Significant decrease in LDH activity was shown in T2 treated group comparing to group T1 at 21 and 42 days of the experiment (Table 5). Highest significant (p<0.05) increment in this parameters was shown at the end of experiment in methionine treated group (T1), methionine plus PSO (T2) comparing to control and group T3. While intubation of pomegranate seed oil to normal animals (T3) caused significant (p<0.05) decrement in serum LD activity comparing to T1 and T2 treated groups. Also the results have clarified that were no significant (p>0.05) differences between T3 and control group in the same duration of treatment when compared in each other.
Table 5: Effect of pometone on serum lactate dehydrogenase activity in methionine overload female rabbits
|
Groups |
Day |
|||
|
T3 (methionine+ PSO) |
T2 (PSO30 mg/ kg. BW) |
T1 (methionine 100 mg /kg. BW) |
Control |
|
|
63.81±0.8 A a |
65.84±1.2 A c |
65.87±0.9 A c |
65.25±1.06 A a |
0 |
|
62.61±1.05 C a |
104.73±0.9 B b |
137.28±0.9 A b |
64.88±1.08 C a |
21 |
|
65.13±1.04 C a |
114.92±0.9 B a |
158.96±0.9 A a |
64.93±1.2 C a |
42 |
Values are mean ± SE, n=8; Small letters denote within group difference p<0.05. Capital letters denote between groups difference p<0.05.
The present study showed a significant elevation in LDH activity in animals treated with methionine for six weeks indicating occurrence of liver function disorder (Ferre et al., 2002; Ebbesen and Ingersity, 2005). The liver produce a large amount of LDH which are secreted to the circulation with injury or death (Wolff, 2006), in addition, release of liver enzyme from cytosol can occur secondary to cellular necrosis with membrane damage (Valentin et al., 1990). This fact is documented in this study by the oxidative stress induced by methionine overload leading to cell damage and increase in this enzyme. Also methionine over load may cause oxidative stress and damaging the hepatocell membrane and central portal liver cells with subsequent release of AST and ALT enzymes (Garcia-Terijano et al., 2001; Sahi et al., 2006). The current study found a marked significant decrease in LDH activity after PSO intubation explained the alleviation of oxidative insults by antioxidants properties of PSO. Some studies showed the presence of flavonoids, steroids, terpenoids and tannins in pomegranate reduce xenobiotic- induced hepatotoxicity in animals and counteract the damaging effects of oxidative stress, cooperating with natural system like glutathione and other endogenous protective enzyme (Abdel-Rahman and Abdel-Megeid, 2006; Toklu et al., 2007). No study, however reviewed the effect of pomegranate seed oil on serum LDH as a hepatoprotective agent. In conclusion, methionine overload induced oxidative stress and pomegranate seed oil can ameliorate the oxidative stress evidenced by measured biomarkers.
CONCLUSION
The study conclude that methionine overload induced oxidative stress and pomegranate seed oil can ameliorate the oxidative stress evidenced by measured biomarkers.
CONFLICT OF INTEREST
There do not exist any conflict of interest.
Authors contribution
Baraa Najim Al-Okaily designed the experiment, analyzed and interpreted the data. Lekaa Najim Abdulla performed the experiment and wrote the paper. Khalisa Khadim Khudair gave technical support and conceptual advice.
Aknowledgement
Authors of this study would like to thank College of Veterinary Medicine, University of Baghdad, Iraq for their support.
REFERENCES





