Advances in Animal and Veterinary Sciences
Research Article
Development of Real-time PCR Assay for Detection of Rotavirus Infection in Diarrheic Bovine Calves
Haq Adil Anamul1, Kuldeep Sharma2, Yashpal Singh Malik3*, Kuldeep Dhama4, Praveen Kumar Gupta5
1Veterinary Officer, Tral, Pulwama, Jammu & Kashmir, India; 2Division of Microbiology, National Institute of Research in Tribal Health (NIRTH), Jabalpur, MP-482003, India; 3ICAR National Fellow, Division of Biological Standardization, 4Division of Pathology, 5Division of Veterinary Biotechnology, Indian Veterinary Research Institute, Izatnagar 243122, Bareilly, Uttar Pradesh, India.
Abstract | Rotavirus infection is widespread and major cause of acute viral gastroenteritis in dairy animals. The study describes development and application of a reverse transcription SYBR green real-time PCR assay system for the detection of group A rotaviruses (RVA) in bovines. The examination of clinical samples (n=80) showed consistent diagnostic specificity and sensitivity of the real-time PCR assay in comparison to a routinely used RNA electrophoresis method. The specificity of the assay was confirmed using other enteric viruses with the melting curve analysis of amplified products. The reproducibility of the assay was proved by low values of coefficient of variation in the intra- (1.7%) and inter-assay (2.4%). In comparison to RNA-PAGE screening method which detected 25% (20/80) positive samples for RVA, the newly developed real-time PCR detected 46.25% (37/80) positive samples for RVA, providing higher sensitivity of the assay developed. Reports on development of real time PCR for detection of RVs in animals are limited and in particular from India are not available for the use of the said test for detection of RVs in bovines. The results indicate use of real-time RT-PCR assay as a method of choice for the specific detection of RVA in field samples and particularly in a situation where a number of other clinically resembling infectious agents like picobirnavirus, coronavirus, calicivirus, and astrovirus are known to coincide as mixed infection.
Keywords | Type A rotavirus, Real-time PCR, NSP4 gene, Melting curve analysis
Editor | Muhammad Munir (PhD), Avian Viral Diseases Programme, Compton Laboratory, Newbury, Berkshire, RG20 7NN, UK.
Received | April 02, 2015; Revised | May 01, 2015; Accepted | May 01, 2015; Published | May 06, 2015
*Correspondence | Yashpal Singh Malik, Indian Veterinary Research Institute, Izatnagar (U. P.), India; Email: malikyps@ivri.res.in
Citation | Anamul HA, Sharma K, Malik YS, Dhama K, Gupta PK (2015). Development of real-time PCR assay for detection of rotavirus infection in diarrheic bovine calves. Adv. Anim. Vet. Sci. 3(6): 321-324.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.6.321.324
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Anamul et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Enteric and respiratory viral infections are among the most common worldwide problems and are being recognized as the major challenge to the livestock sector (Razzaque et al., 2009; Lorenz et al., 2011). Among several causes of acute viral gastroenteritis, rotavirus (RV), a member of the Reoviridae family, is the most prominent cause of neonatal diarrhea worldwide (Estes and Kapikian, 2007), causing up to 25% mortality in young animals (Dhama et al., 2009). Losses assimilate to the farmers through additional expense for enhanced managemental issues, medicines, reduction in optimum animal growth and loss of the calves (Razzaque et al., 2009). RVs have been classified in 8 species (A-H) (Matthijnssens et al., 2012), where species A rotaviruses (RVA) are predominantly present in humans, bovines and other mammalian and avian species throughout the world (Matthijnssens et al., 2012). There are six structural proteins (VP1 to VP4, VP6 and VP7) and six non-structural proteins (NSP1 to NSP6) of which NSP4 has pleiotropic role and has been a target for RV diagnosis (Ciarlet et al., 2000; Estes and Kapikian, 2007).
Rotaviruses of animal origin have shown established potential for interspecies transmission and additionally impose risk for human infection, signifying need for rapid and sensitive method for better case management and detection of RVs early in the course of infection. The common methods adopted for RVA detection includes RNA-PAGE, enzyme immunoassay, latex agglutination test, electron microscopy (EM), and conventional reverse transcription (RT)-PCR (Dhama et al., 2009). Indubitably, the higher sensitivity of RT-PCR improved the detection rate of RVs in clinical samples (Dhama et al., 2009). Additionally, the use of real-time PCR (qPCR) is growing exponentially because it’s several leads over conventional PCR assays, including higher accuracy, sensitivity and specificity, rapidity, which assists in large number of samples in a short span of time (Espy et al., 2006). Among the several chemistries available for real-time PCR assays, SYBR Green based assays are most widely used. Recently, real-time PCR based methods have been reported for detection of many different viruses of veterinary importance such as Foot and mouth disease virus, bluetongue, Newcastle disease virus, avian influenza virus (Aguero et al., 2007).
Real-time PCR assays have been described for detection of human RVs (Zeng et al., 2008) and reports are limited for animals RVs and in particular from India are not available for the use of the said test for detection of RVs in bovines. In the present study, we describe the development and application of a specific SYBR Green based real-time PCR with subsequent melting curve analysis for detection of RVA using a primer set specific to genome segment 10 (NSP4) of bovine RVA strains. As the primer binding site of the NSP4 genes are more conserved over the years (data not shown), for the epidemiologic investigations NSP4 is an important target in determining RV strains circulating in the population.
MATERIALS AND METHODS
Sampling
During the present study, faecal samples (n=80) from diarrheic cases were used to evaluate the usefulness of the SYBR Green based real-time PCR assay for its diagnostic potential. The freshly voided stool samples were obtained from bovine calves (up to the age of 6 months) from different regions of India.
Sample Preparation and Viral RNA Extraction
Suspension of samples was prepared and stored as described in our earlier reports (Malik et al., 2014). Total RNA was extracted from 500 μL of the diarrheic faecal suspension using an equal volume of TriReagent-LS (Sigma-Aldrich, St. Louis, USA). The RNA was eluted finally with Nuclease Free Water (NFW) in a final volume of 25 µL. The isolated RNA was assessed qualitatively and quantitatively using Nanodrop Spectrophotometer (ND-1000, Thermo-Scientific, USA) and stored at -70°C until further use.
RNA-PAGE and Primer Designing
The genome of RV was detected and analyzed by RNA-PAGE following the method described earlier (Malik et al., 2011). The primers for NSP4 gene were designed by using GeneTool Lite 1.0 software (BioTools Inc., Edmonton, Canada) based on the highly conserved region from the sequence data available in the GenBank nucleotide database. The sense (nt position 458-5’ TGGCGAAATAGACATGAC 3’-475) and anti-sense (nt position 567-5’ CGACGGCAGC TCAACCTCTTA 3’-587) primers for 130 bp product length were selected and synthesized in the scale of 0.5 nmole by Metabion GmbH, Martinsried, Germany.
Reverse-Transcription-Polymerase Chain Reaction
Reverse-transcription for cDNA synthesis was carried out following procedures outlined in our earlier report (Malik et al., 2014). A PCR was carried out using the newly synthesized NSP4 primers with an initial denaturation at 95°C for 4 min, followed by 35 cycles of denaturation at 95°C for 30s, annealing at 55°C for 30s, and extension at 70°C for 1 min, and a final incubation at 70°C for 10 min.
Production of Plasmid
The amplified product was run in agarose gel and NSP4 gene specific PCR product of 130 bp was excised from the gel using QIAquick Gel Extraction kit (Qiagen, Germany) and cloned into pJET1.2/blunt cloning vector (Fermentas, Lithuania). The clones were screened for the presence of the insert by Colony PCR and restriction digestion with 40U BglII (Fermentas, Lithuania). The positive plasmid was also confirmed through sequencing and sequence chromatogram was visualized in BioEdit v7.0.5 analysis software (Isis Therapeutics, CA, USA) and Mega Blast was performed with deduced sequences within the non-redundant nucleotide database (http://www.ncbi.nlm.nih.gov/Blast) to confirm the presence of the NSP4 gene specific to RVA.
Real-Time PCR
Real-time PCR reaction conditions including annealing temperature were optimized using NSP4 plasmid construct. The optimum conditions with annealing temperature of 58°C, and primer concentration of 2.5 pMol/µl for the forward as well as the reverse primer gave amplification in all the positive samples with good ∆Rn values, highest sensitivity with negligible formation of primer dimers. The amplification efficiency and slope value of the real-time PCR assay were determined by amplifying duplicate amounts of the serially diluted plasmid DNA (10-7 to 10-0 copies) containing the 130 bp NSP4 gene insert. Real-time PCR was carried out in Strategene Mx3005 Real-Time PCR System (Stratagene, La Jolla, USA) using 1 µL of serially diluted plasmid DNA as a template, 10 µL of DyNAmoTM SYBR Green 2-Step qRT-PCR Master Mix (Finnzymes, Finland) with ROX (0.3X) as passive reference dye. Cycling parameters for the real-time PCR consisted of initial denaturation for 10 min to activate the Hot Start Taq DNA Polymerase, followed by 40 cycles at 95°C for 15s, 55°C for 30s and 72°C for 30s with the fluorescent data acquisition after the annealing and the extension steps. Melting curve analysis was performed for each sample to verify the specificity of each product which consisted of denaturation at 95°C for 1 min, annealing at 48°C for 30s and final denaturation at 95°C for 30s. The fluorescent data acquisition was continuous from annealing to the final denaturation step. The Real time data obtained were analyzed by MxProTM QPCR Software version 4.10 (Stratagene, USA).
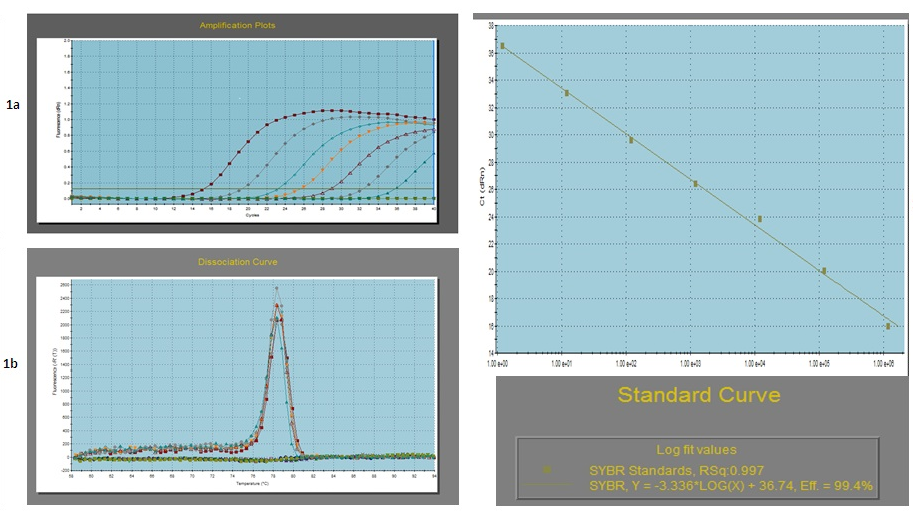
a- Amplification plot of the serially 10 fold diluted plasmid DNA; b- Dissociation curve of the serially diluted plasmid DNA (Tm = 78.35 ± 0.5°C): c- Standard curve of the serially diluted plasmid DNA
Validation of the Assay
To validate the real-time PCR assay, the detection limit and amplification efficiency was determined. Intra-assay and inter-assay variability was determined of each dilution as Mean ± S.D. and coefficient of variation (C.V) (in %) of the threshold cycle (Ct) values. The values of S.D. and C.V. ranged from 0.31 to 0.11 and 4.32% to 1.08% for different dilutions which were within the statistical limits. The standard and dissociation curves for the NSP4 gene are given in Fig. 1. The standard curve showed a slope of -3.336 with a Y intercept of 36.74 with a high regression coefficient (r2) of 0.997. The PCR efficiency was calculated from the slope of the standard curve (E = (10-1/slope-1) x 100) and was found 99.4% with an amplification factor (A) of 2.00. The melting curve showed a melting temperature (Tm) value of 78.35°C (±0.5°C) which was taken as a standard for the clinical samples to be evaluated using this test. Moreover to confirm the RV specificity of the developed approach, samples that were positive for other enteric viruses, including picobirnavirus and RV species B were also included, which gave negative results.
RESULTS AND DISCUSSION
Real-time PCR assay with the optimized conditions was applied to screen clinical samples (n=80) obtained from diarrheic bovine claves with exception of that the template used was 2 µL cDNA instead of diluted plasmid standards. Melting curve analysis was performed for each sample to verify the specificity of the product(s). The samples whose melting curves shared the same Tm with the reference plasmid genotype standard and Ct values below 35 were interpreted as positive. Of the 80 faecal samples, 37 (46.25%) were detected positive with a Ct value of less than 35 and Tm value of 78.35°C (±1.0°C). RNA-PAGE analysis of the same samples showed a percentage positivity of 25% (20/80) with genomic migration pattern of 4:2:3:2 typical for RVA with ‘long’ electropherotype pattern of segments 10 and 11. Hitherto reports confirm use of RNA-PAGE for screening of RVs with percent positivity in the range of 3.3% (Jhala and Raghvan, 1997) in bovine calves from India. All the samples which showed segmented pattern in RNA-PAGE were detected positive in real-time PCR assay. Thus by using real-time PCR, the detection rate of RVs was increased by around 21%. The higher detection sensitivity of the newly developed real-time PCR assay in comparison to RNA-PAGE is indicative of the suitability of this assay for screening of the field samples for bovine RVAs.
Though, to our knowledge, this is the first study in which a real-time PCR detection assay has been developed and applied for detection of bovine RVAs targeting more conserved genome segment 10, this newly developed SYBR Green based real-time PCR for NSP4 gene needs to be further validated with RV detection in other animal species with larger number of samples so as to better define its practical utility in molecular epidemiological studies and designing and adapting appropriate control measures. Detection of positive animals will be useful to monitor the RV infection within animal population and provide an early warning signal to predict an impending epidemic and risk for human population. In addition, the RVA specific real-time PCR developed in this study will be a useful tool for differential diagnosis of RV in a situation where a number of other agents like coronavirus, calicivirus, astrovirus etc. co-exist and are clinically indistinguishable.
ACKNOWLEDGEMENTS
The authors are thankful to the Director, Indian Veterinary Research Institute for providing funds to carry out the present work. Special thanks to the staff and attendants from animal farms and hospitals who helped during collection of samples.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES