Advances in Animal and Veterinary Sciences
Review Article
Recent Advances in Immunotherapy for the Treatment of Cancer
Namrata Acharya, Dhruv Gohel, Vinod Kuberkar, Senthilkumar Natesan*
Western Range Biopharmaceuticals Pvt Ltd, Cluster Innovation Centre, Genome Research Centre, Maharaja Sayajirao University of Baroda, Vadodara, Gujarat 390 002, India.
Abstract | Immunotherapy based approaches to modulate the body’s own immune defense mechanism to fight cancer is gaining momentum due to its successful application for treating different types of cancers recently. There are four major types of immunotherapy approaches including nonspecific immunotherapy using immune-modulatory molecules, monoclonal antibody therapy, vaccines, and cellular immunotherapy. The use of antibodies for treating cancers has been well established there are about dozen antibodies currently available in clinical practice for the treatment of various cancers. Approval of Dendritic cell based therapy by regulatory authorities has led to the recognition of immune cells as drug for treatment of clinical disease. Other modes of immune cell based therapies including Natural killer cells and T cells are also under various stages of development. This review is aimed to provide an overview of different modes of immunotherapy and its application in human and animals.
Keywords | Immunotherapy, Dendritic cell, NK cells, Monoclonal antibodies, Cancer
Editor | Muhammad Munir (DVM, PhD), Avian Viral Diseases Program, Compton Laboratory, Newbury, Berkshire, RG20 7NN, United Kingdom.
Special Issue | 4(2015) “Advances in animal disease diagnosis, vaccines and therapeutics”
Received | January 23, 2015; Revised | February 21, 2015; Accepted | February 23, 2015; Published | March 13, 2015
*Correspondence | Senthilkumar Natesan, Maharaja Sayajirao University of Baroda, Vadodara, Gujarat, India; Email: senthil.natesan@wrbio.com
Citation | Acharya N, Gohel D, Kuberkar V, Natesan S (2015). Recent advances in immunotherapy for the treatment of cancer. Adv. Anim. Vet. Sci. 3(4s): 23-29.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.4s.23.29
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Acharya et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Immunotherapy for cancer is a rapidly advancing field of translational science having enormous therapeutic application potential for cancer. Cancer immunotherapy has been recognized as ‘breakthrough of the year 2013’ by the journal Science and it was also recognized by release of a special issue on cancer immunotherapy by the journal Nature recently.
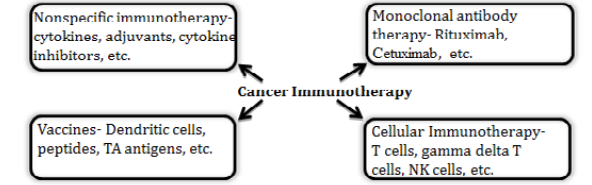
There are four major areas of cancer immunotherapy- nonspecific immunotherapy, monoclonal antibody therapy, vaccines, and cellular immunotherapy as depicted in the Figure 1. Nonspecific immunotherapy includes use of cytokines and other chemicals that stimulate a general immune response or remove the blockades of immune activation against cancers. Monoclonal antibodies are used for tagging the cancer cells for immune mediated killing or altering the cell signalling pathways to inhibit proliferation or inducing apoptosis. Cancer vaccines include dendritic cells based vaccines where these antigen presenting cells were loaded with tumour associated antigens ex vivo and then injected back in to the patients. The cellular immunotherapy approach utilizes ex vivo expanded specific population of immune cells (T-cells, NK cells, gamma delta T cells etc.) for the treatment of cancer. Some of these approaches are approved by US FDA (Food and Drug Administration) and some are still in the experimental stages. Several clinical trials are undergoing worldwide by several academic & research institutions and companies to bring novel therapies to cancer patients based on cancer immunotherapy approaches.
History of Cancer Immunotherapy
Though exponential advancement has happened in the field of immunotherapy during the last decade, this field of science originated about a century back in 1891, when the surgeon William Coley started administering a particular type of bacteria (now known to be Streptococcus pyogenes) to the cancer patients following his observation that some cancer patients get cured following a bacterial infection. Important discoveries in the field of immunology including discovery of tumor specific antigens, discovery of interferons, discovery of monoclonal antibody, discovery of other several subset of immune cells including Dendritic cells, discovery of T cell antigen receptors, etc. led to the evolution of cancer immunotherapy and development of various US FDA approved novel therapies for cancer.
Nonspecific Immunotherapies
The discoveries of cytokines including Interferon alpha (IFN-a) and Interleukin-2 (IL-2) had led to the successful application of these molecules for cancer therapies. The cytokines have the ability to regulate the expression several immunological components including major histocompatibility complex antigens, immunosuppressive peptides, proto-oncogenes or endogenous cytokine production, cell proliferation or apoptosis and they bring the therapeutic effect through these functional mechanisms. More than twelve cytokines have been tried either through direct administration or gene therapy approaches. The cytokines have also been used as adjuvants for many anti-cancer vaccines to strengthen vaccine-induced immunity and to modulate the immune responses (Dhama et al., 2013). Due to the immunostimulatory nature and anti-tumour activity, some cytokines are directly used for treating cancers. For example, IL-2, an immunostimulatory cytokine induces T cell immunity against cancer. There are several FDA approved therapeutic cytokines for the treatment of specific cancers. For example, Interferon (IFN)-α2a is used in patients with hairy cell leukaemia and IFN-α2b is also used for the therapy of AIDS-related Kaposi’s sarcoma, hairy cell leukaemia, follicular lymphoma, melanoma, and multiple myeloma. IL-2 is approved for the treatment of metastatic melanoma and renal cell carcinoma. The use of recombinant granulocyte colony stimulating factor (G-CSF) and recombinant granulocyte macrophage colony-stimulating factor (GM-CSF) in cancer patients has also been widely used as supportive immunotherapy (Vacchelli et al., 2013; Vacchelli et al., 2014). Anti-cytokine molecules are also used as anti-cancer drugs due to the role of certain cytokines negatively regulating immunity and promoting cancer growth. For example, anti-IL10 or anti-TNFa therapy falls into this category. Recently, the use of recombinant canine G-CSF was found effective for treatment of neutropenia in dogs, which accelerated recovery and decreased the severity of neutropenia (Yamamoto et al., 2011).
Monoclonal antibody therapy
The use of antibodies for treating cancers has been established as one of the most successful strategies over the past two decades. There are about dozen antibodies approved by FDA and currently available in clinical practice for the treatment of various cancers and several additional antibodies are being tested in early and late stage clinical trials. For example, the antibodies Trastuzumab, Bevacizumab, Cetuximab, Panitumumab, Rituximab targeted against the antigens ERBB2, VEGF, EGFR, EGFR, CD20, respectively are used successfully for cancer therapy. Several protein and carbohydrate molecules expressed on the surface of cancer cells are potential target antigens for developing novel antibody based therapies. Ideally, the antigen targets should be specifically expressed on the cancer cells but not on the normal cells or it could be expressed at higher levels by cancer cells than by normal cells. This will lead to selective binding of antibodies to cancer cells. Receptors of the cancer cell surface are very attractive target for antibody therapies as the antibody binding to the receptors lead to inhibition of normal signalling pathway which could lead to cell survival and proliferation (e.g., cetuximab and pantumumab, which inhibit the epidermal growth factor receptor). The antibodies could also be used for delivering drugs for specific targeted killing of cancer cells by conjugating the antibody to a radioactive particle, immunotoxin, or chemotherapeutic agent (e.g., 90Y-ibritumomab tiuxetan and 131I-tositumomab, radionuclide-coupled anti-CD20). The conjugated antibodies guide the effector therapeutic molecule to target cancer cells and also help their internalization to mediate the function. These mAbs also mediate different other functions including activation of the immune system against tumour cells by stimulating immune effector mechanisms such as antibody dependent cell cytotoxicity and complement dependent cytotoxicity (e.g., rituximab, a naked anti-CD20) and interfering with the tumour-stroma interaction (e.g., bevacizumab, which blocks the vascular endothelial growth factor) (Galluzzi et al., 2012). Apart from targeting antigens by different approaches as mentioned above, antibodies are being developed to remove the blockage that is causing suppression of the anti-cancer immunity against cancer cells. For example, the antibodies against the CTLA4 and PD-1 molecules are targeted to remove the blockade for T cell activation and remove the exhaustion of T cells following prolonged activation, respectively (Scott et al., 2012; Leach et al., 1996; Brahmer et al., 2010). The efficacy and safety of therapeutic antibodies depend on nature of antigen (cancer cell specificity, abundance, surface expression, minimal secretion, internalization of antigen-antibody complex etc.) targeted through an antibody. Though there are some clinical toxicities observed during clinical trials of antibody therapies like fever, rash, nausea, bronchospasm, dyspnea, hypotension or tachycardia, anaphylaxis, serum sickness, increased creatinine, headache, etc. the antibody based therapy is a routine practice nowadays in the oncology clinics and used successfully for the treatment of various cancers.
Apart from the wide use of antibodies for cancer therapy in human, few therapies are being developed for treating cancers in animals. For example, Aratana therapeutics, a pet health company based in Kansas City, USA is developing antibody therapies for canines. In 2012, Canine-specific monoclonal antibody against CD20 (AT-004), received conditional approval from the USDA for the treatment of B-cell lymphoma and it was licensed to Novartis Animal Health Inc. for commercialization in United States and Canada. Aratana’s second canine lymphoma monoclonal antibody- AT-005 (against CD52) also received conditional approval for the treatment of T-cell lymphoma in dogs. These therapies in canines are directed against the same molecular targets for which biologic or biosimilar drugs are currently available for treating lymphoma in human (http://www.aratana.com). Similarly, a “caninized” version of anti-EGFR antibody, can225IgG, generated by fusing the variable region gene of 225 (murine precursor of Cetuximab) with canine constant heavy gamma and kappa chain genes is also under development for clinical application (Singer et al., 2014).
Dendritic cell therapy
Dendritic cells (DC) are the most potent antigen presenting cells of different animals that educate the T cells for adaptive immunity. DC was first discovered in mice by Prof. Steinman in 1975, who was awarded Nobel Prize for his discovery and contribution towards the advancement of this field. There are three different types of DCs- myeloid, plasmacytoid, and follicular. Myeloid DCs are the well-studied dendritic cells. The maturity and activation status of DCs are critical for their function. The immature DCs have high capacity of antigen uptake, expressing high level of intracellular MHC-II molecule, low level of co-stimulatory molecules, and limited cytokine secretion ability. Maturation requires additional signals such as microbe associated molecular patterns, damage associated molecular patterns, cytokines, and co-stimulatory molecules. Mature DCs exhibit increased level of MHC-II on the surface, decreased antigen uptake capacity, expression of chemokine receptor for migration, and increased ability to secrete cytokines. Immature DCs play important role in maintaining peripheral tolerance to self-antigens by deletion of antigen-specific T cell clones (clonal deletion) and the expansion of CD4+CD25+FOXP3+ regulatory T cells (Galluzzi et al., 2012; Vacchelli et al., 2013). The monocyte-derived myeloid DCs are used for the treatment of cancer and a FDA approved therapy using monocyte-derived DC is also available for the patients with hormone resistant metastatic prostate cancers. There are several clinical trials ongoing worldwide validating the therapeutic application of DCs for different types of cancers. Different approaches of using DC-based immunotherapy for cancer involves use of ex vivo activated autologous DCs without loading antigens, ex vivo tumour associated antigen loaded autologous DCs, and in vivo antigen loading of DCs. Dendritic cells from different animals including bovine, equine, avian species have been well characterized (Siedek et al., 1997; Renjifo et al., 1997).
NK cell therapy
Natural Killer (NK) cells were first described in 1975 as a distinct subset of lymphocytes, which are larger in size than T and B lymphocytes containing distinctive cytoplasmic granules (Kiessling et al., 1975 and Herberman et al., 1975). They also showed profound tumor cell killing property without any need for prior immune sensitization of the host or antigen presentation. NK cells are characterized by expression of surface markers CD56 (NCAM-1) and CD16 (FcRIII) in human phenotypically. Based on the expression of the T cell marker CD3, they can also be divided into NK cells (CD56+CD3-) and NKT cells (CD56+CD3+). The NKT cells can also be further divided into CD4+ NKT cells and CD8+ NK cells. The markers can be slightly variable among different species. Functionally, NK cells are capable of killing as well as inducing tolerance depending on their activation state. In normal steady state, they express both activating and inhibitory receptors, where the resulting effect is depending on the balance of engaged activating and inhibitory receptors. CD158 is an inhibitory receptor prevents the killing of host cells by engaging with MHC-1 molecules. Similarly, NKG2A is also an inhibitory receptor, which binds to CD94. NKG2D is an activating receptor, which also engages with CD94. Evaluation of the expression of CD158, NKG2A and NKG2D would be important to predict the potential effect of NK cell infusions while administering them for the treatment of cancers (Terme et al., 2008 and Romagné et al., 2011).
NK cells play critical roles in cellular host immunity against cancer and the cancer cells also develop mechanisms to escape NK cell mediated immunity. Current NK cell-based cancer immunotherapy approaches uses the adoptive transfer of either expanded autologous NK cells or stable allogeneic NK cells and NK cell lines or genetically modified of fresh NK cells or NK cell lines to overexpress cytokines, Fc receptors and/or chimeric tumor-antigen receptors (Cheng et al., 2013). Adoptively transferred ex vivo expanded activated autologous NK cells have been shown to improve clinical responses without any obvious adverse side effects in metastatic renal cell carcinoma (RCC), malignant glioma and breast cancer patients. MHC class I expression in cancer cells appear to suppress full cytotoxic potential of autologous NK cells in vivo (Escudier et al., 1994; Ishikawa et al., 2004; Demagalhaes-Silverman et al., 2000). Therapeutic strategies using adoptively transferred allogeneic NK cells and permanent NK cell lines (eg. Human NK-92) have been found successful for cancer immunotherapy. NK cells also recognize Ab-coated target cells and trigger NK cell-mediated antibody dependent cell cytotoxicity resulting in rapid killing of cancer cells. Due to this property, the NK cells have been administered along with several monoclonal antibodies (Cheng et al., 2013).
Gamma delta T cell therapy
Gamma delta T cells (γδ T cells) are a specialized small subset of CD3+ T cells having distinct T-cell receptor (TCR) on their surface. They are different from the vast majority of other T cells harbouring αβ TCR. They also lack the CD4 or CD8 molecules on their surface. In general, the γδ T cells are very low (1-5%) in blood compared to αβ T cells in blood, but they are in highest abundance at the gut mucosa in mice and human. The representation of γδ T cells could be different in different animals. γδ T cells are bridge between innate and adaptive responses due to their functional adaptive ability to rearrange T cell receptor (TCR) genes to produce functional diversity, maintain a memory phenotype, and their innate ability to use their TCR as a pattern recognition receptor to recognize the conserved antigens of pathogens and abnormal cells. Due to these features, γδ T cells have the ability to respond quickly to common molecules produced by microbes and phagocytize them. Human γδ T cells comprise several subsets of cells defined by their T cell receptor. The most prominent one is Vγδ9Vγδ2 γδ T cells. The activated γδ T cells are very attractive for cell based cancer therapies due to their capacity to infiltrate tumours, exhibit MHC-unrestricted cytotoxicity for killing cancer cells, and ability to secrete different cytokines. γδ T cells recognize the phosphoantigens for their activation. The synthetic phosphoantigen BrHPP or the drug zoledronate have the ability to selectively activate human TCRVγ9Vδ2+ γδ T cells and the γδ T cells activated and expanded using these molecules in combination with cytokines have been widely used for cancer therapy (Groh et al., 1999; Viey et al., 2005; Corvaisier et al., 2005; Burjanadze et al., 2007; Bryant et al., 2009; Lamb et al., 2009; Bonneville et al., 2010; Gertner-Dardenne e al., 2012).
In general, the γδ T cell based cancer therapy relies upon either in vivo activation of patients with phosphoantigens/IL-2 or on the adoptive transfer of ex vivo activated and expanded autologous γδ T lymphocytes. The γδ T cell based therapy is safe generally, however, the higher doses of stimuli co-injected with IL-2 increase the level of γδ cell expansion and the frequency of drug-related toxicity including thrombophlebitis, thrombosis, hyperglycemia, hypocalcemia, chest and musculoskeletal pain, gastritis, myocardial infarction and renal creatinine toxicity. In human, the maximum tolerable doses for BrHPP and zoledronate while administering along with IL2 are 1500 and 4 mg/m2 (every 28 days), respectively (Wilhelm et al., 2003; Dieli et al., 2003; Lang et al., 2011; Naoe et al., 2010; Kobayashi et al., 2007; Kobayashi et al., 2011; Kondo et al., 2008; Abe et al., 2009; Fournie et al., 2013).
Adoptive T cell Therapy
Adoptive cell transfer (ACT) therapies involve T-cell stimulation ex vivo by activating and expanding autologous T-cell populations to large numbers using conventional activation/expansion protocols and then transferred back in to the patient (Luca et al., 2006). The earliest method of adoptive T cell therapy was done in early 1980s by using the lymphokine activated killer (LAK) cells, which are generated following IL2 mediated expansion of peripheral blood T cells. The clinical use of LAK cells for cancer treatment showed mixed response in the clinical trials and its wider clinical use never gained momentum due to toxicities and expansion of undesirable T cells that are negatively correlated with tumour regression. Later, the engineered T cells have been applied clinically for the treatment of cancers. The first use of engineered T cells for cancer treatment started in the early 1990s and this approach has led to the successful generation of engineered T cells that express chimeric antigen receptors (CARs) which recognize specific targets on cancer cells. CARs are genetically modified T cell receptors that confer the specificity of an antibody to T cells for a specific cancer antigen recognition and killing of cancer cells. The engineered CAR molecule typically consists of a piece of single-chain variable fragment of monoclonal antibody linked to cytoplasmic domain of signal transduction molecule inside the T cell. The antibody portion helps the T cell to find its target antigen and the cytoplasmic signal transduction domain provides the necessary signals for the activation of T cells, and then the activated T cells effectively proliferate and attack cancer cells (Grupp and June, 2011; Goldstein et al., 2012). The recent versions of engineered CAR molecules also contain co-stimulatory signaling molecules like CD28 or 41BB for providing the potent activation of CAR T cells.
Conclusion and future directions
Cancer immunotherapy is a rapidly advancing field of science promising targeted anticancer effect against several cancers. It has developed enough to find applications in human patients. However, its application in the treatment of cancers in animals is in the early stages due to several reasons such as the cost of therapy, required expertise, investments, infrastructure, etc. Still, it is now being used in increasing number for treatment of pet animals, and hopefully, it will be applied widely in other animals in near future. Persistent scientific effort is need of the hour to bring these novel immunotherapies in veterinary medicine. In addition, the application of immunotherapy in veterinary medicine can also lead to development of novel animal models so that various cellular immunotherapies can be tested prior to use in both humans and animals.
References