Advances in Animal and Veterinary Sciences
Review Article
Zoonotic Pathogens Transmitted from Equines: Diagnosis and Control
Sandip Kumar Khurana1*, Kuldeep Dhama2, Minakshi Prasad3, Kumaragurubaran Karthik4, Ruchi Tiwari5
1National Research Centre on Equines, Hisar, 125 001, Haryana, India; 2Division of Pathology, 4Divison of Bacteriology and Mycology, Indian Veterinary Research Institute, Izatnagar, Bareilly, 243 122, Uttar Pradesh, India; 3Department of Biotechnology, College of Veterinary Sciences, LUVAS, Hisar, 125 004, Haryana, India; 5Department of Veterinary Microbiology, College of Veterinary Science and Animal Husbandry, Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu Chikitsa Vigyan Vishwa Vidyalaya Evam Go-Anusandhan Sansthan (DUVASU), Mathura (UP) – 281001.
Abstract | The diseases of equines and other animal species that are shared between animals and humans come under the category of zoonotic diseases, and always put threat to veterinarians, animal handlers, animal health personnel and general public. These pose a greater threat to pregnant women, infants, children, immunocompromised and old persons, individuals with stress of antibiotic therapy, and other susceptible humans. Equines also play an important role in transmitting several zoonotic diseases causing human illnesses such as those caused by encephalitic alphaviruses, hendravirus, West Nile virus and equine rabies, salmonellosis, glanders, anthrax, methicillin resistant Staphylococcus aureus (MRSA) infection, brucellosis and Rhodococcus equi infections, therefore acts as substantial global health threat. Among these zoonotic threat agents, anthrax and glanders are also potential biological weapons and at times have been used as bio-terroristic agents also. The emergence and re-emergence of equine zoonotic pathogens have been observed from time to time. Antibiotic resistance is a hot topic around the globe at present and certain strains like MRSA, extended spectrum beta lactamase producing Enterobacteriacea can also be transmitted between horses and humans. These drug resistant strains pose greater threat to human beings. Rapid detection of the causative agents of zoonosis, close attention to personal hygiene, identification of potential fomites and vectors, and the use of protective clothing, newer therapeutics and vaccines may contribute to reduce the risk of zoonoses. Equines are used for antivenom and antitoxin production against various antigens, therefore the serum being obtained from equines should be properly screened for various pathogens either by serological methods or molecular assays which are specific for detection of zoonotic agents. The present review discusses several important aspects of zoonotic diseases of equines with special focus on the recent advances in their diagnosis and control.
Keywords | Equine zoonotic diseases, Diagnosis, Control
Editor | Ruchi Tiwari, College of Veterinary Sciences, Department of Veterinary Microbiology and Immunology Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu Chikitsa, Vigyan Vishvidhyalaya Evum Go-Anusandhan Sansthan (DUVASU), Mathura (U.P.) – 281001, India.
Special Issue | 2 (2015) “Reviews on Trends and Advances in Safeguarding Terrestrial /Aquatic Animal Health and Production”
Received | January 20, 2015; Revised | February 10, 2015; Accepted | February 12, 2015; Published | February 16, 2015
*Correspondence | Sandip Kumar Khurana, National Research Centre on Equines, Hisar, Haryana, India; Email: sandipkk2003@yahoo.co.in
Citation | Khurana SK, Dhama K, Prasad M, Karthik K, Tiwari R (2015). Zoonotic pathogens transmitted from equines: diagnosis and control. Adv. Anim. Vet. Sci. 3(2s): 32-53.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.2s.32.53
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Khurana et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Zoonotic diseases, naturally transmitted between vertebrate animals and man, are highly variable on the basis of their severity and transmissibility. The “one medicine concept” involving a convergence of animal, human and environmental science professionals for prevention, control and eradication of cross-species disease transmission is gaining momentum, where zoonoses has assumed central position (Dhama et al., 2013a; Mukarim et al., 2015; Plowright et al., 2015). The emergence and re-emergence of zoonotic diseases poses a greater threat to pregnant women, infants and children, immunocompromised and old persons, persons under antibiotic therapy stress, veterinarians, animal handlers and animal health personnel (Stull et al., 2012).
Zoonoses constitute nearly 60% of all known human infections and over 75% of all emerging pathogens. They are caused by a diverse group of microorganisms and infectious syndromes caused by zoonotic pathogens are equally diverse. Classification of zoonoses is broadly based on the nature of the pathogen, animal host, severity of disease and mode of transmission between animals to humans. Zoonoses have major implications on economics, labour and health productivity globally. Preventing and controlling zoonoses is even more critical today in the context of globalization of international trade, changes in agricultural practices and global warming (Dhama et al., 2013b). They are responsible for affecting productivity of both humans and animals severely, thus contributing to aggravation of poverty.
Equines are used for various purposes like riding, racing, sports, draught, transport, ceremonies, antitoxin/antibody production, etc., throughout the world (Burnouf et al., 2004). There remains a contact between the human and these equines at various stages which pave way for the spread of various diseases of equines to human and hence human beings may acquire equine zoonotic bacterial, viral or other infectious diseases either directly or by indirect means. The number of diseases affecting horses and other members of the Equidae family carries zoonotic potential including viral, bacterial, rickettsial, anaplasma associated, fungal and parasitic infections, which will be elaborated through this review. Zoonotic diseases may occur as mild transient infection to severe lethal or highly contagious infection and thus can be a suitable candidate of bioterrorism also under certain circumstances. Veterinarians have a key role in revealing various emerging zoonotic infections due to their close involvement with both animals and owners. Growing possibilities of exploring zoonotic pathogens as bioterrorism agents is another worry which reflects the main functioning of veterinarians in early detection of bioterrorism-associated outbreaks of zoonotic diseases. Antivenom are commonly produced from equine serum, so critical attention should be given during this time as this can be a major source for the transmission of dangerous pathogens to human (Lalloo and Theakston, 2003; Theakston et al., 2003). Inference accredited to equine zoonoses depends on prevalence of particular disease along with its case-fatality rate in human population. Encephalitic alphaviruses, hendravirus, equine rabies, salmonellosis, glanders, anthrax, MRSA infection, brucellosis and Rhodococcus equi are among the very important zoonoses. Weese (2002) has reviewed the risk of zoonotic diseases to veterinary practitioners with emphasis on occupational aspects. The present review covers several important diseases which may be transmitted through equines to man or vice-versa with emphasis on their epidemiology, diagnosis and control calling for emergent need of collaborative approach of medical doctors, horse breeders, veterinarians, other governmental and non-governmental agencies for early preparedness; however there could be several others which have not been described here.
ZOONOTIC PATHOGENS TRANSMITTED FROM EQUINES VIRAL PATHOGENS
Encephalitic Alphaviruses
Western equine encephalitis virus (WEEV), eastern equine encephalitis virus (EEEV) and Venezuelan equine encephalitis virus (VEEE) are common zoonotic encephalitic alphaviruses. These have been isolated from horses, humans, mosquitoes, birds, rodents and some other animals also. These are vector borne and transmitted through blood sucking arthropods, thus considered as arboviral infection and condition is referred as Arboviral Encephalitis including EEE, WEE, VEE and WNV as a cause of encephalitis in horse (Strauss et al., 1995; Smith et al., 1997; Kapoor et al., 2010). Ordinarily arboviruses are not directly transmitted from horses to humans under usual circumstances, though aerosolization could be one possible risk factor. As per the recommendations of the Centers for Disease Control and Prevention (CDC), suspected clinical specimens such as cerebrospinal fluid and serum must be handled under level 2 biocontainment facility equipped laboratories with limited entrance. Lundstrom (2014) has reviewed progress in alphavirus vector development and vaccine technology which enabled clinical trials in humans.
Eastern Equine Encephalitis Virus (EEEV) Infection
EEEV was first isolated from horses in 1933 (Giltner and Shahan, 1933; TenBroeck and Merrill, 1933). The virus is propagated in nature between birds and mosquitos. The virus is considered to be most virulent among the alphaviruses causing encephalitis with case-fatality rate of up to 70% in human beings (Zacks and Paessler, 2010). EEEV infections has greater significance American continent where it was responsible for more than 180 cases in human (Aguilar et al., 2007). Mosquito vectors have a role in the transmission of the virus form horses from human. The incubation period is 4-10 days, fever, headache, vomiting, respiratory distress, seizures and coma may occur in human encephalitis cases. The mortality rate in horses is higher than in WEEV infection. Diagnosis is done by ELISA, haemagglutination-inhibition, neutralization assay and virus isolation. Formalin inactivated vaccine is used in horses as double vaccine with WEEV. Honnold et al. (2014) demonstrated that chimeric live-attenuated EEEV vaccine candidates protest mice against a lethal aerosol challenge. Trobaugh et al. (2014) have reviewed recent advances in alphavirus virulence mechanisms that could be used to design live-attenuated vaccine against EEEV/ alphaviruses.
Western Equine Encephalitis Virus (WEEV) Infection
WEEV was first isolated from brain of a horse (Meyer et al., 1931). First human WEEV was confirmed in 1938. WEEV is naturally propagated in enzootic cycle between passerine birds and specific mosquito vector. Some rodents and lagomorphs also act as reservoir hosts (Pfeffer and Dobler, 2010). Horses and humans are dead end hosts (Go et al., 2014). WEEV infections generally have an incubation period of 2-7 days with early non-specific symptoms like fever, anorexia, headache, nausea and vomiting. Diagnosis of WEEV infection is done by ELISA, haemagglutination-inhibition and neutralization assay (Martin et al., 2000). RT-PCR has also been developed for its diagnosis (Linssen et al., 2000; Lambert et al., 2003). Formalin inactivated vaccine is used in horses as double vaccine with EEEV.
Venezuelan Equine Encephalitis Virus (VEEV) Infection
VEEV is propagated in nature in enzootic cycle between rodents and mosquito vector (Taylor and Paessler, 2013; Carossino et al., 2014; Go et al., 2014). Epidemics occur when mosquitoes transmit the virus to humans and equids (Tigrett and Downs, 1962; Walton et al., 1973). Horses, donkeys and mules have sufficient viremia and are able to transmit the infection through mosquitoes (Young, 1972; Mackenzie et al., 1976). Some reports suggest that humans are also able to transmit the infection especially in urban settings (Watts et al., 1997, 1998; Morrison et al., 2008). Madsen et al. (2014) demonstrated that inhibition of Ago2, an important component of RNA-induced silencing complex (RISC), resulted in decreased replication of encephalitic alphaviruses and thus may be a future therapeutic. A live attenuated vaccine TC83 is thought to available option for humans as well as horses (Engler et al., 1992). However, formalin inactivated whole viral vaccines are also available (Taylor and Paessler, 2013). DNA based vaccines are also available which can be produced rapidly and are cost effective (Dupuy et al., 2011; Tretyakova et al., 2013; Carossino et al., 2014). Encouraging results have also been obtained with chimeric vaccines (Paessler et al., 2003; Paessler and Weaver, 2009; Carossino et al., 2014).
Japanese Encephalitis (JE)
Japanese encephalitis (JE) is very important and common mosquito borne flavivirus causing encephalitis and is regarded a major public health problem in Asian countries (van-den Hurk et al., 2008, 2009; Pawaiya et al., 2010a). The disease affects primarily human beings, horses and pigs. The infection in horses is usually subclinical with signs of pyrexia, depression, tremors and ataxia. Abortions and stillbirths are common manifestations in pigs.
JE virus was first isolated from a case of fatal human encephalitis in Japan in 1935 (Lewis et al., 1947). In human beings incubation period is 5 to 15 days with majority of cases being asymptomatic. Encephalitis is reported in about 0.04% cases only. Severe rigors, pyrexia and malaise are non-specific symptoms lasting one to six days. Signs of encephalitis are neck rigidity, cachexia, hemiperesis, convulsions and pyrexia. There is only one serotype of JEV and four genotypes (Chen et al., 1990, 1992; Tsarev et al., 2000). Urbanization, population spurt in tropical areas, increased transportation and global warming are responsible for spread of infections to newer areas (Go et al., 2014).
JEV is transmitted by Culex tritaeniorhynchus, C annulus, C annulirostris and Aedis mosquitoes (Rosen, 1986). Pigs and aquatic birds are amplifying hosts that have high titre viremia which acts as a source of infection for mosquitoes (Rosen, 1986). Humans and horse do not have sufficient viremia to transmit the infection and thus are dead end hosts. Yeh et al. (2010) developed a duplex reverse transcriptase PCR for rapid differential detection of west nile and japanese encephalitis viruses which is rapid, sensitive and specific and is useful both in humans and horses. Yeh et al. (2012) developed a diagnostic algorithm to serologically differentiate west nile virus from japanese encephalitis virus infection and its validation in field surveillance of horses.
Earlier inactivated vaccines were used. A purified vaccine from vero-cell adapted SA 14-14-2 strain has been developed (Tauber et al., 2007). Another chimeric vaccine containing pr M and E proteins of JEV has also been developed and found to show high level of immunogenicity (Guy et al., 2010; Halstead and Thomas, 2011). Singh et al. (2015) demonstrated JENVAC, a Vero-cell derived vaccine with a long lasting, broadly protective immunity.
West Nile Virus (WNV) Infection
West Nile virus (WNV), a member of the Flavivirus genus of the Flaviviridae family, is one of the most widely distributed mosquito–transmitted arbovirus having potential global public health concerns (Gulati et al., 2014). WNV was first isolated in Uganda in 1937 (Smithburn et al., 1940). There have been outbreaks in Africa, the Middle East, Asia, and Australia before spread to USA and Canada (Weaver and Barrett, 2004). The infection is maintained in nature between Culex mosquitoes and birds, whereas horses, humans and other mammals are dead end hosts (Blitvich, 2008; Dhama et al., 2010a; Beck et al., 2013; Go et al., 2014). Birds like crow are also affected by this viral infection (Mishra et al., 2012). Molaei et al. (2010) have studied the vector and host interactions governing the epidemiology of WNV in Southern California. Most of the WNV infections are subclinical and only less than 1% humans develop neurologic disease (Mostashari et al., 2001; Hayes et al., 2005; Hayes and Gubler, 2006; Porter et al., 2011).
Yeh et al. (2010) developed a duplex reverse transcriptase PCR for rapid differential detection of West Nile and Japanese encephalitis viruses which is useful both in humans and horses. Yeh et al. (2012) could serologically differentiate West Nile from Japanese encephalitis by a diagnostic algorithm. Currently, there is no suitable therapy for WNV infection (Paterson et al., 2011). A number of vaccines have been explored for horses including inactivated whole West Nile virus (West Nile–Innovator®, Vetera® WNV vaccine) and chimeric recombinant canarypoxvirus - Recombitek® Equine WNV Vaccine (De Filette et al., 2012; Gulati et al., 2014). DNA vaccine for WNV has been licensed in USA, and with the use of prime boost approaches it may protect WNV (Kumaragurubarn and Kaliaperumal, 2013), but such vaccine (West Nile–Innovator® DNA) has recently been discontinued by Pfizer (Brandler and Tangy, 2013; Gulati et al., 2014).
Hendra Virus (HeV) Infection
Hendra virus (HeV) was first isolated in 1994 from an outbreak among humans and horses (Selvey et al., 1995; Murray, 1996). This is one of deadliest human and veterinary pathogen causing respiratory and encephalitic illness in humans with high mortality rate which may exceed 70% (Dhama et al., 2010b; Croser and Marsh, 2013). Hendra virus (HeV) is a zoonotic paramyxovirus in the genus Henipavirus. There had been 48 outbreaks with increasing number of outbreaks with each passing year since it was first reported (Aljofan, 2013). Horses acquire infection from flying foxes. Clinical signs in horses include fever, anorexia followed by respiratory signs that include frothy nasal discharge. Human beings get infection through direct contact with secretions from infected horses. No evidence of human to human, human to horse and flying fox to human has been reported (Selvey et al., 1996), however, Williamson et al. (1998) have shown the role of fruit bats, horses and cats in transmission of Hendra virus. Flying fox bats acts as reservoirs and horses may encounter the infection from contaminated secretions or excretions through environment. Veterinarian get the disease while physical examination of oral cavity of horses. Human have flu like symptoms with mainly respiratory signs. The diagnosis of this infection is based on ELISA (IgG and IgM), RT-PCR and isolation of virus. There are no effective therapeutics against HeV infections and protective measures while examining horses are the only way to minimize the risk of its spread (Mahalingam et al., 2012). Human monoclonal antibody against HeV glycoprotein (G) protein is considered to be most promising passive immunotherapy (Bossart et al., 2011).
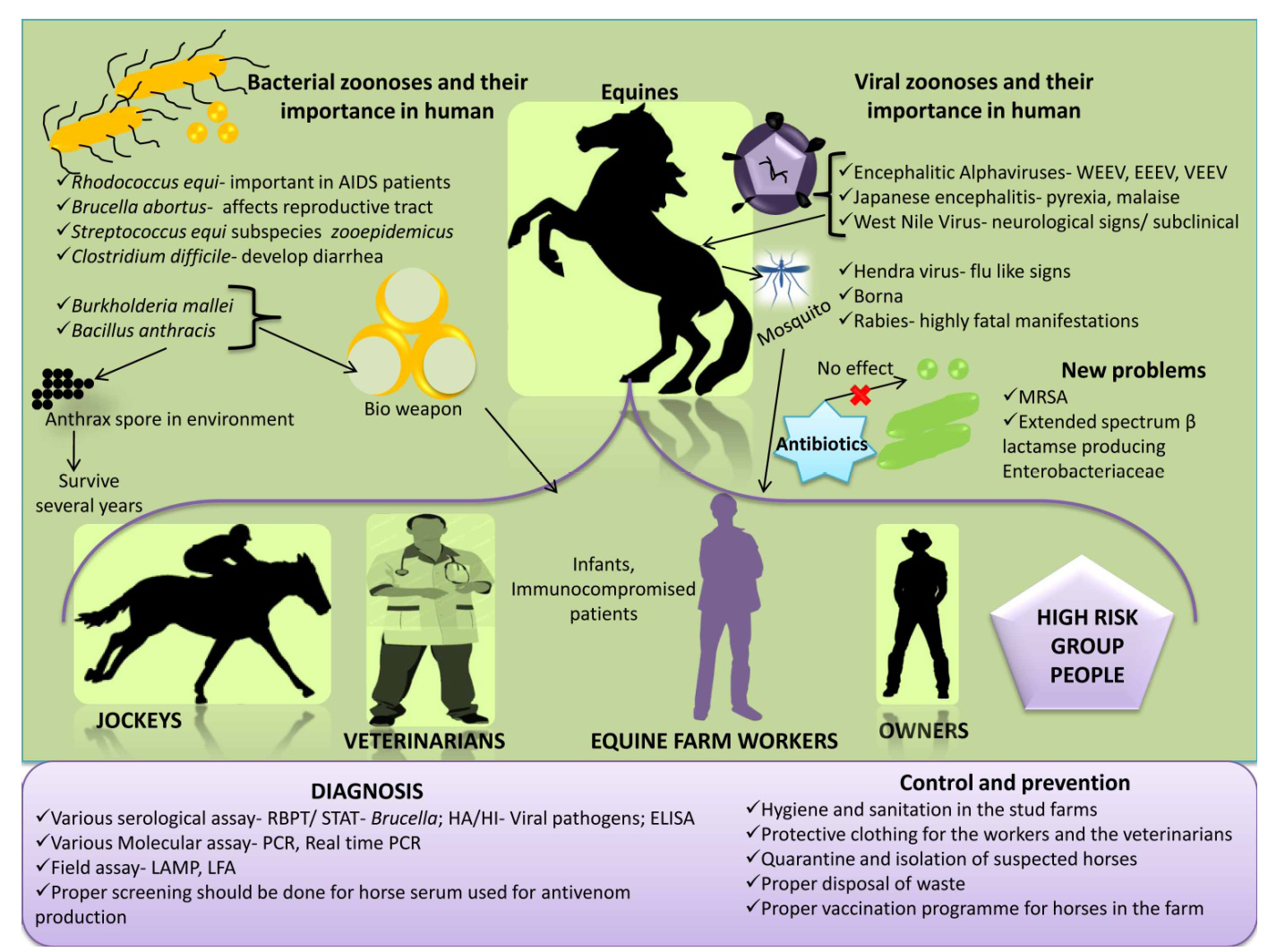
Figure 1: An overview on equine zoonoses
Equine Rabies
Rabies is a well-documented zoonotic disease that causes huge fatalities throughout the world. Rabies is a fatal neurological disease of mammals which is not an exception in equines, affects horses, mules and donkeys (Pawaiya et al., 2010b). It is caused by Lyssavirus of Rhabdoviridae family. The major animal involved is the dog and affected dogs can transmit the virus through their saliva. Inspite of low incidences in horses, literature reveal documented reports of equine rabies in past years as 82 reports of rabies in 1998, 65 cases in 1999 and 52 reports of equine rabies in 2000 have been from United States. Rabies in horse’s do not have the typical symptomps as in dog but the animal seems alert, lack of muscular in-coordination and seizures are common (Hudson et al., 1996). Though as compared to small animal practice probability of acquiring rabies from equines in veterinarian is low but possibility cannot completely be ignored due to severity of infection and variation in expression of symptoms. In equines, among furious and dumb form, paralytic or dumb form is more reported with the signs of rubbing of site of wound, gradual lameness and colic (Krebs et al., 2000, 2001; Weese, 2002). Animal handlers, veterinarians and horse owners are at highest risk for the transmission of rabies virus to them. Humans experience various symptoms like ataxia, loss of awareness, paralysis of muscles, etc. Vaccines are available for animals and humans that can prevent rabies, however history of vaccination does not necessarily exclude the risk of rabies, as one report described that even among 21 vaccinated horse, 5 animals could acquire the rabies (Green et al., 1999). Initial diagnosis can be made by differentially diagnosing all undifferentiated neurological diseases or encephalitis from Rabies. Animals may die due to cardiac arrest within 2-7 days or 14 days after appearance of clinical symptoms as nerves are affected in this disease. In infected animals, central nervous system components, saliva and salivary glands all are rich source of rabies virus hence any contact between infected saliva and breached skin or mucous membrane of horse or handler, jockey, owner must be avoided by taking biosecurity precautions. If any case is observed or suspected, higher authorities must be notified.
An overview on equine zoonosis is presented in Figure 1.
BACTERIAL PATHOGENS
Rhodococcus equi Infection
Rhodococcus equi is a gram positive, aerobic soil actinomycete responsible primarily for severe respiratory disease of young foals with high mortality rate (Prescott, 1991; Yager et al., 1991; Khurana et al., 2009; Giguere et al., 2011a, b; Khurana, 2014; Khurana et al., 2014; Khurana, 2015). R. equi also causes extra-pulmonary complications in equines including enteritis, arthritis and abscesses in abdomen (Giguere and Prescott, 1997). R. equi was first recovered from lung of a foal as Corynebacterium equi (Magnusson, 1923) and was reclassified as R. equi (Goodfellow and Alderson, 1977). This organism is emerging as an important pathogen in AIDS patients (Weinstock and Brown, 2002), drug therapy (Mizuno et al., 2005) and some other immunosuppressive conditions (Napoleao et al., 2005). The most common manifestation of human R. equi infections is pneumonia, others include fever, diarrhoea, abscesses in various internal organs and arthritis.
Virulence associated protein A (Vap A), a cell surface lipoprotein is essentially required for virulence in foals whereas virulence associated protein B (Vap B) is often associated with disease in human beings and pigs. Intracellular localization of R. equi is responsible for its prolonged and difficult therapeutic management. No suitable serodiagnostic test or vaccination is available for R. equi infection of equines as well as humans till date (Khurana, 2015).
Human beings acquire infection mainly through inhalation of dust harboring bacteria, from domestic animals including equines and wound, however man to man transmission is thought to be rare (Weinstock and Brown, 2002).
Agar gel diffusion test developed by Nakazawa et al. (1987) and ELISA by Giguere et al. (2003) have not been found to be of much promise for diagnosis of R. equi serologically. In India, diagnosis through post-mortem examination (Garg et al., 1985; Saxena and Narwal, 2009) and isolation of R. equi from clinical samples (Khurana et al., 2009). Various PCR assays have been developed (Sellon et al., 2001; Arriaga et al., 2002; Ladron et al., 2003; Oldfield et al., 2004; Ocampo-Sosa et al., 2007; Pusterla et al., 2007; Letek et al., 2008; Monego et al., 2009). The most valuable diagnostic procedures are combination of cultural methods along with PCR assay (Khurana, 2015).
Rifampicin along with macrolides is the drug of choice for treatment of R. equi infection. Rifampicin resistance have been reported which is posing a challenge in therapy (Asoh et al., 2013; Burton et al., 2013; Goldstein, 2014; Liu et al., 2014). Proper management and sanitation at farms is very important for control of disease at equine farms. Hygiene is important in immunocompromised human beings.
Anthrax
Anthrax is caused by Bacillus anthracis, an extremely resistant spore forming bacteria. Horses generally get infection by grazing in areas contaminated by anthrax. The typical incubation period is 3 to 7 days. Bacteria multiply and disseminate throughout the body through blood and lymphatic system, and release lethal toxin causing cell death and breakdown of tissues.
Symptoms appear very rapidly which include high fever, agitation, chills, colic, anorexia, dullness, laboured breathing and seizures. Bloody diarrhoea, swelling around the neck may also be observed. Chest, abdomen and genitals may also get swollen. Death may occur in 2 to 3 days after occurrence of first symptoms. This is controlled by vaccination in endemic areas, early diagnosis and treatment, burning and burial of dead animals.
Horses are usually less susceptible than ruminants, but reports of outbreaks of anthrax are available in horses from Minnesota and North Dakota. Horses may have prolonged course of disease. Affected horses exhibit symptoms of marked pyrexia, colic, dyspnea, and subcutaneous edema and death may occur abruptly.
Human beings contract infection from contaminated horses and their accessories and are often associated with three forms cutaneous, gastrointestinal and inhalation (Hicks et al., 2012). One more form of injectional anthrax has also been reported (Lalitha et al., 1988; Beaumont, 2010; Booth et al., 2011; Jallali et al., 2011). Spores of anthrax bacilli are so sturdy that they can survive for years in the environment and that can cause major problem for human population.
Control of anthrax is done through vaccination, early detection and reporting, quarantine, antibiotic therapy of exposed animals, burning or burial of dead animals that had suspected or confirmed anthrax infection. Vaccination of horses is done only in endemic farms and areas.
Glanders
Glanders is a contagious and fatal disease of horse, mules and donkey with zoonotic potential (Malik et al., 2010; Varga et al., 2012; Verma et al., 2014a). This is a disease known since ancient times and was identified in 4th century BC by Hippocrates (Colahan et al., 1999). The disease is caused by a non-spore forming gram negative bacillus called Burkholderia mallei. Most common mode of transmission of this organism is inhalation and ingestion of contaminated feed and water. The disease occurs in chronic form in horses, where bacteria are found in nasal discharges and skin lesions (OIE 2004, 2008), whereas in mules and donkeys the disease occurs in acute form (Hunting, 1913; Gulati and Gautam, 1962). The acute form of glanders involves pulmonary, cutaneous and nasal sites (Jubb et al., 1993), and is characterized by pyrexia, cough, discharges from nostrils, ulcers on nasal mucosa and nodules on the skin finally leading to death.
Chronic form in horse also shows all three clinical manifestations which include pulmonary, nasal and cutaneous forms. The human glanders is not very common, but it is having very high mortality rate of 90-95% in untreated septicaemic infection and 50% in treated humans. Human outbreaks have not been reported.
This organism is very important from biological warfare angle due to high rate of mortality and ability of small number of organisms to establish the infection. The diagnosis of glanders is done by isolation and allergic test (Mallein test) which is prescribed test for international trade. The test is not very specific, so it should be used along with complement fixation test which is also prescribed for international trade. The test is not very specific, so it should be used along with complement fixation test (CFT), which is also prescribed for international trade. Various serological tests for diagnosis of glanders include complement fixation test (CFT), indirect haemagglutination assay (IHA) and ELISA. Singha et al. (2014) have developed an indirect ELISA using truncated TssB protein for serodiagnosis of glanders.
Various PCR assays have been developed (Grishkina and Samygi, 2010; Zhang et al., 2012) which have been found to be rapid, sensitive and specific. Janse et al. (2013) developed a multiplex qPCR for detection and differentiation of B. mallei and B. pseudomallei. Diagnosis in human beings is generally done by CFT and imaging studies.
No vaccine is available for prevention of glanders for animals or humans (Burtnick et al., 2012). In case of death suspected due to glanders, the carcass should not be opened and must be buried deep or incinerated.
Brucellosis
Brucellosis is one of the most important disease problems of animals and human beings. This is caused by Brucella abortus in equines, and is manifested by fistulous withers, poll evil, lameness due to joint infection and rarely late abortions in mares. Horizontal transfer of Brucella spp. to horses from cattle and pigs has been documented (Forbes, 1990). Brucellosis is considered to be an occupational disease from public health point of view that mainly affects slaughter house workers, butchers and veterinarians. This causes undulant fever in human beings.
Ehizibolo et al. (2011) have reported a sero-prevalence of 14.7% of equine brucellosis in Nigeria. Tahamtan et al. (2010) have reported a sero-prevalence of 2.5% for brucellosis in horses in Iran. A sero-prevalence of 5.88%, 12.89% and 5.78% has been reported from Egypt (Montasser et al., 1999), India (Sharma et al., 1979) and Pakistan (Ahmed and Munir, 1995a, b), respectively.
The incubation period in humans varies from 1-3 weeks. The symptoms may include irregular fever, headache, weakness, malaise, profuse sweating especially during night. Coughing, chest pain, irritation, insomnia, depression are occasionally encountered. In many patients, the symptoms last for 2 to 4 weeks and are followed by spontaneous recovery. Others develop recurrent bouts at 2-14 day intervals. Most people with this undulant form recover completely in 3 to 12 months. A few patients become chronically ill, with symptoms of chronic fatigue, depressive episodes and arthritis. Relapses can be seen months after the initial symptoms, even in successfully treated cases. Occasional complications include arthritis, endocarditis, granulomatous hepatitis, meningitis, uveitis, orchitis, cholecystitis, osteomyelitis, and rare cases of encephalitis. Asymptomatic infections are also common in humans.
Diagnosis relies on the detection of circulating antibodies followed by the bacteriological isolation and serum agglutination tests [Rose Bengal plate agglutination test (RBPT) and standard tube agglutination test (STAT)]. Molecular diagnostic assays can be used for detection instead of serological tests because serological assays like RBPT, STAT have the disadvantage of false positive reaction against other gram negative bacteria (Karthik et al., 2014a). Various molecular assays like polymerase chain reaction (PCR), Real time-PCR, etc., can be employed for detection of the pathogen. Recent field assays like loop mediated isothermal amplification assay and lateral flow assay can be employed (Karthik et al., 2014a, 2014b).
Brucellosis being intracellular requires the need for antibiotics that can target intracellular pathogen. Treatment in humans can be done with combination of various antibiotics like doxycyline, streptomycin and rifampicin. Combinational therapy is followed to reduce the infection quickly and also to reduce the toxicity (MacMillan et al., 1982; Yousefi-Nooraie et al., 2012).
Salmonellosis
Salmonellosis is an enteric disease with clinical manifestation of chronic diarrhea, fever which may turn into acute toxic enterocolitis or septicaemia in various vertebrate hosts including horses and human beings. Etiological agent is Gram negative bacteria of Salmonella genus with multiple serotypes. Horses of all age groups are susceptible. Multidrug resistant S. Typhimurium DT104 (Salmonella enterica subspecies enterica Serotype Typhimurium Definitive Type 104) has been recovered from many horses in Ontario and has significant zoonotic potential due to its high lethality rate in human beings as well. Initially S. Typhimurium DT104 was isolated from cattle in 1988 in England and Wales but consequently was reported from sheep, pigs, poultry and horse. Fecal-oral route with high inoculums size containing more number of bacteria is the most common way for zoonotic spread of salmonellosis in human population, though in immunocompromised persons low inoculums may also produce the disease. Higher mortality rate and resistance to commonly preferred antibiotics further aids to the potential of this pathogen for zoonotic transmission. Suspected cases should be monitored and treated separately. Strict follow up of personal hygienic measures and proper disinfection of stables, contaminated equipments and utensils will help in reducing the menace of zoonotic transmission (Fone and Barker, 1994; Weese et al., 2001a; Weese, 2002).
Streptococcus equi Subspecies zooepidemicus Infection
Streptococcus equi subspecies zooepidemicus is considered opportunistic pathogen in horse, but causes infection in cattle, sheep, goat, pig and dog also. S. equi subspecies zooepidemicus has more than 98% DNA sequence homology with S. equi subspecies equi. Pelkonen et al. (2013) have shown that S. equi subspecies zooepidemicus is transmitted from horse to human beings and they found that human and equine isolates were identical or closely related. Lindahl et al. (2013) reported an outbreak of repiratory disease due to S. equi subspecies equi. Downar et al. (2001) have documented infection of Streptococcal meningitis from close contact with an infected horse (Downar et al. (2001)).
S. equi subspecies zooepidemicus is β-haemolytic streptococci in Lancefield group C (Lancefield, 1933). The equine Lancefield group C streptococci are differentiated biochemically by their ability to ferment sorbitol, lactose and trehalose (Quinn et al., 1994). In addition, PCR can be used to genetically identify different species and subspecies (Alber et al., 2004; Baverud et al., 2007; Preziuso et al., 2010).
Clostridium difficile Infection
Clostridium difficile (C. difficile) is an anaerobic causative agent for colitis in horses and human (Jones et al., 1987; George et al., 1978; Weese et al., 2001b). C. difficile associated diarrhoea can be mild, self-limiting or peracute leading to fatal outcomes in horses. It affects all ages of horses ranging from neonatal to adults. In acute cases of diarrhea rapid diagnosis is based on detection of bacterial toxins in fecal samples. C. difficile is a well-recognized pathogen of human also. The infection in man varies from mild to severe pseudomembranous colitis leading to intestinal perforation and death. Transmission between animals and humans has been poorly studied. Equines suffering with C. difficile diarrhoea should be considered infectious, particularly to people undergoing antimicrobial or chemotherapeutic treatment. Proper precautions (gloves, gowns and boots) and close attention to personal hygiene may prevent the chance of zoonotic transmission. Sporicidal disinfectant (5–10% bleach solution) should be used to clean the contaminated equipment and the areas under use. The veterinarians who develop acute diarrhoea following contact with suspected or confirmed case of infection in a horse should take medical help to confirm the infection.
Methicillin Resistant Staphylococcus spp.
In the era of antibiotic resistance, methicillin resistant Staphylococcus aureus (MRSA) is one of the prime concerns. Different Staphylococcus spp. can harbour horses of which S. aureus and its variant MRSA are the important species as they can be transmitted to humans and can cause major infections. In the early phase of MRSA, concern was restricted to humans and community associated MRSA which were increasing throughout the world. Livestock associated MRSA has spread recently among animals (Hartmann et al., 1997; McCarthy et al., 2012). Though the report of MRSA in horses was late but there are arrays of documentation after its first report in later part of nineteenth century (Stull et al., 2012). Literature reveal that approximately 10% of healthy vigorous horses cart MRSA in their nasal passages, intestinal tracts and on their skin, thereafter these colonized horses act as reservoirs of MRSA in the community which are efficiently capable of transmitting MRSA in the human population in and across the globe due to frequent international movement and trading of horses (Weese et al., 2005, 2006). Most of the methicillin resistant Staphylococcus organisms affect soft tissues and joints in horses but these are resistant to treatment with antibiotics. Some instances pneumonia, metritis and sinusitis are also documented in horses (Smiet et al., 2012). There is always danger of transfer of these drug resistance bacteria from animals to humans, and these methicillin resistant organisms can jump both ways from human to animal and also from animal to human (Weese and Lefebvre, 2007). Veterinarians top the list of humans suffering from this methicillin resistant Staphylococcus spp. transmission form horses (van Duijkeren et al., 2011). Bacteriophage therapy has yielded good response against MRSA (Karthik et al., 2014c). Similarly, broad spectrum beta lactamase producing Enterobacteriacea can also be transmitted from horses to human (Boyen et al., 2013).
Tuberculosis
Tuberculosis (TB) affects different mammals and many variants including extremely drug resistant TB has emerged which poses serious threat to human beings (Karthik, 2012; Karthik et al., 2013). Incidences of TB are sparse in horses mainly due to established control programmes, however few workers have reported the disease as confirmed by the presence of causative agent (Pavlik et al., 2004). TB is a zoonotic disease caused by Mycobacterium tuberculosis, M. bovis, and members of M. tuberculosis complex in several mammalian hosts including horses though they are considered comparatively resistant. Horses residing in close proximity with infected cattle acquire the infection as evidenced from the earlier reports showing presence of M. tuberculosis and M. bovis as well and advocate a possibility of interspecies transmission and zoonotic prospective of M. tuberculosis. Horses infected with pulmonary TB illustrate lesions of multiple tuberculoid granulomas in lung with manifestation of granulomatous lymphadenitis in mediastinal and tracheobronchial lymph nodes, which can be further confirmed by laboratory culture techniques and quantitative real-time PCR (Keck et al., 2010; Blahutkova et al., 2011; Konstantin et al., 2012).
PARASITIC PATHOGENS
Cryptosporidiosis
Equine cryptosporidiosis is caused by a protozoal pathogen, Cryptosporidium parvum, causing enteric disease in several species including humans and is most commonly associated with foals and immunodeficient animals. Infected horses shed oocysts with the shedding rate of 0–21% which have infective potential, horses develop asymptomatic cryptosporidiosis. High prevalence of disease with 71% infection rate is documented in foals and zoonotic transmission of Cryptosporidium from foal to handling veterinarians is also reported (Coleman et al., 1989; Cole et al., 1998). Humans express symptoms of copious watery diarrhea which may lead to critical grave condition. In immunocompromised persons, the disease exists in a self-limiting form. Zoonotic implications occur due to high shedding rates, hence precautions must be taken while dealing with diarrhoic animals to minimize the risk of zoonosis (Snyder et al., 1978; Konkle et al., 1997; Majewska et al., 1999; McKenzie and Diffay, 2000).
Giardiasis
Fecal shedding of Giardia oocysts from horses is an indication of zoonotic risk. Giardiasis caused by Giardia intestinalis is the most common intestinal parasitic disease characterized by mild or severe diarrhea. Zoonotic transmission of Giardia is supported by feco-oral route. Asymptomatic shedding of Giardia in 25% of adult horses and 71% cumulative infection rate in foals further suggest possibility of zoonotic potential of this pathogen in horses besides other species (Xiao and Herd, 1994). Fecal cyst detection and ELISA are available for diagnosis for giardiasis (Rishniw et al., 2010).
Other Zoonotic Diseases of Equines
Several important zoonoses shared between man and equines have been described above, however all the possible zoonotic threats related to equines are not detailed and still there are many others like Borna virus, Nipah virus infection as geographically limited zoonotic, dourine, crytosporidisis (Sellon, 2007; Xiao, 2008), giardiasis, leptospirosis, dermatophytosis, Halicephalobus gingivalis (Micronema deletrix) under certain specific conditions, are also among the several other infections capable of being transmitted from equines to human beings thus adding to the disease burden. Besides viral and bacterial pathogens, parasitic, protozoan and fungal agents are also involved in equine zoonoses. Leptospirosis is another important disease of public health concern that can be transmitted from various animals. Leptospirosis is a zoonosis of global concern caused by spirochetes of genus Leptospira (Ebani et al., 2012). Leptospira spp. is endemic in various parts of the world. Various serovars like Pomana, Icterohaemorrhagiae and Bratislava are recorded in horses but risk of zoonotic transmission of leptospirosis from horses is not immense. Hall and Bryan (1952) and other workers have reported leptospirosis in horse, which harbor the infection as accidental host (Hall and Bryan, 1952; Hogg, 1974; Barwick et al., 1998). Symptoms in horse include anorexia, fever, lethargyness, renal dysfunction, jaundice; abortion and still birth can also occur in pregnant mares (Divers et al., 1992; Timoney et al., 2011). Equine recurrent uveitis or moon blindness occurs after weeks or months after the onset of systemic leptospirosis in horses. Symptoms in human include jaundice, fever, muscular pain, vomiting, uveitis etc. (Hartskeerl et al., 2004; Verma et al., 2013). Microscopic agglutination test is the gold standard test for diagnosis of leptospirosis but several disadvantages makes it difficult to perform. Various recombinant proteins are used in ELISA and latex agglutination assay formats for diagnosis of leptospirosis which are effective in diagnosis (Deneke et al., 2014). Dermatophytosis (ringworm) is a fungal dermatologic disease of zoonotic concern affecting variety of animals including horses, caused by species of Microsporum or Trichophyton. Horses are mainly affected by T. equinum with the clinical presentation of mild or subclinical form of disease to severe lesions imitating to pemphigus foliaceus. Dermatophytosis can be transmitted from horses to persons in contact through direct and indirect routes (Pascoe, 1976; Pier and Zancanella, 1993; Huovinen et al., 1998).
Preparedness and strategic planning to counter equine zoonosis
To protect the public and animal health of equine sector from devastating effects of zoonoses, equine emergency preparedness plans are required to be implemented by collaboration of local, regional, state officials, academic institutions, tribal and other allied government agencies to safeguard the equine industry and personnel involved by providing public education, following integrated surveillance plans, epidemiological disease investigations and prevention strategies on zoonotic threats of already mentioned emerging equine zoonotic diseases. As swiftly as possible any suspected incidence should be detected and controlled, and containment of the incidence should be arranged to protect the health and environment for stabilizing the economy. Local veterinary surgeon and state personnel should assure educating equine veterinarians, owners, trainers and farm managers about equine industry organizations and effective disease management practices.
In the current era of increasing global population, globalization trends, tourism expansion, ecosystem and biodiversity changes like global warming, emerging drug resistance, need for effective therapeutics and vaccines, immune stresses, we need to strengthen research and development programmes, implement strategic and planned veterinary and medical approaches, the one world health one medicine concept, multi-disciplinary and international level surveillance /networking employing geographical information system (GIS), early warning systems and to tackle emerging/re-emerging infectious diseases of animals and their increasing zoonotic and pandemic risk (Slingenbergh et al., 2004; Kahn et al., 2007; Jones et al., 2008; Bergquist, 2011; Dhama et al., 2013a, 2013b, 2013c, 2014a; Tiwari et al., 2013; Verma et al., 2014b). Important vectors and reservoirs of infectious zoonotic pathogens need to be brought under control to prevent the spread of infectious agents and disease risks and threats to equines and related public health concerns (Daszak et al., 2000; Bengis et al., 2004; Zinsstag et al., 2007; Dhama et al., 2013d). Recent advances in diagnostics and molecular detection tools for delivering rapid and confirmatory diagnosis of zoonotic infectious pathogens of animals and affecting humans need to be explored to their full potential, including PCR, real-time PCR, multiplex PCR, LAMP, recombinant protein based diagnostics, biosensors, biochips, microarrays, gene sequencing, phylogenetic analysis and nanodiagnostics (Schmitt and Henderson, 2005; Belak, 2007; Belak et al., 2009; Bollo, 2007; Ratcliff et al., 2007; Balamurugan et al., 2010; Bergquist, 2011; Deb and Chakraborty, 2012; Dhama et al., 2012, 2014b; Ayyar and Arora, 2013). Apart from conventional killed and live vaccines, due priority need to be given for developing effective and safer new generation prophylactics comprising of DNA vaccines, plant based (edible) vaccines, reverse genetics vaccines, vector vaccines, protein/peptide vaccines, gene deleted mutant vaccines, reassortant vaccines, chimeric vaccines, virus like particles (VLP), vaccine cocktails, and vaccine delivery systems (oral, spray administration) (Meeusen et al., 2007; Dhama et al., 2008, 2013e; Koff et al., 2013). Adaptation of regular and judicious vaccination strategies, DIVA strategy, prime boost regimens and giving booster vaccinations must be implemented appropriately. Emphasis should be given in utilizing potential of novel and alternative/complementary immunomodulatory and treatment regimens comprising of immunotherapy, cytokine therapy, si-RNAs, avian egg antibodies, toll like receptors, phages, enzybiotics, probiotics, nutritional immunomodulation, herbs and nanomedicines for devising appropriate prevention and control programmes to counter infectious pathogens including zoonosis (Mahima et al., 2012; Dhama et al., 2013f, 2014a; Malik et al., 2013; Tiwari et al., 2014). RNA interference has been used successfully in various parasitic infections which can be adopted for other diseases (Sudhakar et al., 2013). Implementation of good management practices, strict biosecurity rules, proper hygiene and sanitation procedures, follow up of isolation and quarantine, and trade restrictions need to be taken care of very timely for checking and controlling the transmission and spread of zoonotic pathogens. This holistic approach would pave road to lower the disease incidences / outbreaks as well as in controlling the zoonotic pathogens of equines and their public health concerns.
CHALLENGES AHEAD AND FUTURE OUTLOOK
Lack of awareness, inadequate communication between veterinarians and public health organizations, and weak surveillance systems for zoonoses are the major problem areas. Infections that affect animals and humans fall in ‘no man’s land’. In most human-animal episodes, neither the human nor the veterinary health systems have the capacity to deal with the outbreaks. Strengthening of risk assessments and early warning systems, laboratory capacity for diagnosis, monitoring and treatment are the much needed priorities. Monitoring and testing of all antivenom or antitoxin produced from horse serum against various pathogens like Brucella, Streptococcus, Burkholderia mallei and other viral pathogens using different specific diagnostic assays can prevent spread of zoonotic diseases. Zoonotic illnesses can infect humans by entering the body in a variety of ways: animal bites, insect bites, by ingestion, by inhalation, through cuts/scratches and through the eyes or contact with other mucous membranes. A combination of precautions including breaking the transmission cycle especially in arboviral infections is effective in preventing zoonotic infections. Appropriate follow up of early diagnosis; maintain high personal hygiene and precautions while handling animals, fomites, tissues and various specimens effectively reduces the probability of disease transmission to a great extent. Physicians, veterinarians and public health professionals must work together to recognize and control zoonotic diseases. Approaches to the control of zoonoses differ according to the type of zoonoses, because majority of direct and cyclozoonoses and some saprozoonoses are most effectively controlled by techniques involving the animal host, and methods used to combat these diseases are almost entirely the responsibility of veterinary medicine. The control of metazoonoses may be directed at the infected vertebrate host, at the infected invertebrate vector or both. National and international agencies with mandates to control zoonotic diseases should coordinate their activities and share resources to accomplish prevention and control of zoonotic diseases. The key success to prevent and control equine zoonotic pathogens lies in optimum utilization of recent developments in advances in diagnostics, surveillance /networking, vaccines, therapeutics and good management practices. Proper hygienic and sanitary practice while handling horses or any other animals can prevent spread of infectious agents not only from animals but also vice versa. Veterinarians who are at most risk should wear protective clothings as gloves, mask, boots etc., while examining animals to prevent spread of zoonotic infection.
REFERENCES




