Advances in Animal and Veterinary Sciences
Research Article
Clinical Study on Dermatophytosis in Calves with in vitro Evaluation of Antifungal Activity of Bergamot oil
Wagdy Rady El-Ashmawy1, Ekbal Abd EL Hafez2, Haithem Abd El Saeed1
1Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 2Microbiology Department National Organization of Drug Control and Research, Egypt (NODCAR).
Abstract | Ringworm is a fungal and zoonotic infectious disease, caused by different species of dermatophytes. In this study, skin scrapings and hair samples were collected from beef calves recently introduced into a beef farm they have clinical signs of dermatophytosis. The collected samples were directly examined for fungal elements by direct microscopy and fungal culture. Fungal culture revealed Trichophyton verrucosum. The antifungal activity of Bergamot oil (Citrus Bergamia) alone or in combination with salicylic acid using different concentrations was evaluated in vitro and revealed that Bergamot oil with different dilutions (1.25%, 2.5% and 5%) has a very effective antifungal effect against Trichophyton verrucosum.
Keywords | Bergamot oil, Trichophyton verrucosum, Antifungal activity, Dermatophytes, Beef calves.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 11, 2014; Revised | November 26, 2014; Accepted | November 27, 2014; Published | December 16, 2014
*Correspondence | Wagdy Rady El-Ashmawy, Cairo University, Giza, Egypt; Email: ubiowagdy@staff.cu.edu.eg
Citation | El-Ashmawy WR, Abd EL Hafez A, Abd El Saeed H (2015). Clinical study on dermatophytosis in calves with in vitro evaluation of antifungal activity of Bergamot oil. Adv. Anim. Vet. Sci. 3(1): 34-39.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.1.34.39
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 El-Ashmawy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Ringworm in cattle is a highly contagious skin infection all over the world. It is an infection of the superficial, keratinized structures of the skin and hair of man and animals. The disease is caused by a group of keratinophilic filamentous fungi called dermatophytes in the Genera Trichophyton, Microsporum and Epidermophyton (Gudding and Lund 1995). Trichophyton verrucosum is the most common etiologic agent of cattle (El-Diasty et al., 2013).
Animals are infected by contact with arthrospores (asexual spores formed in the hyphae of the parasitic stage) or conidia (sexual or asexual spores formed in the “free living” environmental stage). Transmission between hosts usually occurs by direct contact with a symptomatic or asymptomatic host (Murray et al., 2005). It had been reported that housing animals in close proximity to each other for long periods in the presence of infected debris was responsible for the high incidence of the disease in winter (Al-Ani et al., 2002). However, the disease appears to be more common in tropical than temperate climates, and particularly in countries or areas having hot and humid climatic conditions (Radostits et al., 2007).
The typical lesion in cattle presenting 10 to 50 mm patches of hair loss, desquamation and crust formation usually confined to the head, neck and, sometimes, other parts of the body surface is a heavy, greyish-white crust that is raised perceptibly above the skin, affecting young calves’ more than adult cattle (Cam et al., 2007; Akbarmehr, 2011). Cattle ringworm is rapidly spread in the herd via infected propagules (hyphae and specialized fungal spores named arthrospores). The disease is responsible for great economic losses due to skin injuries and many casualties in animal products (wool, meat, etc.) as reported by Weber (2000). Dermatophytosis had been considered the most common zoonosis worldwide, affecting more children than adults (Achterman and White, 2012; Adeleke et al., 2008).
MATERIALS AND METHODS
Animals in the Study
The study was carried out from September 2013 till 2014 (Autumn and Winter) in a beef farm at Giza governorate. The farm contains 250 beef calves (local mixed breeds) of 6-9 months age. Skin lesions appeared on 30 animals after 2 weeks of receiving calves.
Skin lesions also appeared on hands and face of 2 workers in the farm in contact with diseased animals.
Samples Collection
The samples of skin scraping and hair were collected from the periphery of the lesion after cleaning with 70% ethyl alcohol in sterile falcons. Samples were collected from infected animal’s suffering from lesions suggesting ring worm infections (Circumscribed areas of hair loss filled with raised white scales on head, neck or all over the body). The study aims to isolate and identify the causative agent of ring worm from the affected calves and to evaluate the antifungal activity of Bergamot oil in vitro.
Examination of Samples for Fungi
Direct Microscopical Examination using KOH (20%)
One or two drops of 20% KOH (potassium hydroxide) were placed on a microscopic slide and a small amount of the specimen was added and then, the slide was gently passed through a low flame and covered by a cover slip. After 2 h, the specimen was examined for the presence of arthrospores and hyphae under a light microscope according to Ellis et al., 2007.
Isolation and Identification of Dermatophytes from Samples
Scrapings of skin and hair were reduced in size to pieces approximately 1 mm across and the hair roots were cut into similarly sized fragments. Each sample was cultured on the surface of Sabaroude Dextrose Agar (SDA) containing chloramphenicol. The culture media were incubated at 25°C and 30°C for up to 5 and 21 days. After isolation the cultures were transferred to freshly prepared SDA media to obtain pure cultures. Pure cultures were also maintained in SDA slants at 5±1°C. The obtained dermatophytes were identified by their cultural morphology and microscopic characteristics. Microscopic identification of positive fungal cultures was carried out using the method described by Murray et al. (2005); Chessesbrough (2006). Briefly, a drop of lactophenol cotton blue stain was placed on a clean glass slide. A portion of mycelium was transferred into the lactophenol cotton blue stain and teased with a 22 gauge nichrome needle to separate the filaments. Cover slip was placed on the preparation and examined under low and high power magnification using a much reduced light for identification.
Identification of Dermatophytes
The identification was based on colonial appearance, pigment production and the micro morphology of the spores produced. Cultures were examined at 4 or 5 days intervals from the onset. Some characteristics were also noted on the texture, colour and shape of the upper thallus and the production of pigment on the underside as described by Ellis et al. (2007); Cowen, (1990); Rohde and Hartmann (1980).
In vitro Antifungal Assays
The following procedure was used according to Magaldi et al. (2004). Fungal spores were harvested after 7 days old on SDA slant. Culture was washed with 10 ml normal saline in 2% Tween 80 with the aid of glass beads to help in the dispersion of the spores. The spore suspensions were standardized to 105 spores/ml. SDA was prepared according to specifications, autoclaved at (121°C for 15 minutes) and supplemented with 0.05% chloramphenicol and dispensed into 11 cm diameter Petri dishes. One ml of each standardized spore suspension (105 spores/ml) was evenly spread on the surface of the SDA plates. Then, by using a special instrument known as sterile cork porer (6 mm in diameter), wells were made on the surface of SDA plates. Using Vernier callipers for measuring zone of inhibition.
Determination of Minimum Inhibitory Concentrations (MIC)
MICs for the Bergamot oil were established using an agar dilution method (Banes-Marshall et al., 2001). Before the addition of the oils, 0, 5% (v/v) of Tween 20 was first added to the agar. Use of Tween 20, an emulsifier, enhances the solubility of oils in agar up to a final concentration of 4% (v/v) with oil, although at concentrations higher than this the oil will not solubilize even with Tween 20 incorporated into the agar. Initial experiments demonstrated that the incorporation of Tween 20 only into the agar had no effect on the growth of the bacteria under test.
Therefore controls had no oil nor Tween incorporated in the agar.
Test Compounds and Susceptibility Testing Assays
Natural essence of bergamot produced by the Consorzio del Bergamotto of Reggio Calabria, Italy, were supplied by Bergamon S. r. l. (Rome, Italy).
Mold inoculum preparation
All molds were subcultured from stock cultures onto plates of either Sabaroude dextrose. These plates were then incubated at 30°C until a lawn had developed over the entire plate. The inoculums were prepared by aseptically cutting the lawn growth into 10x10 mm squares.
Plate preparation and analysis
The agar dilution method described above was modified for filamentous fungi by substituting either malt extract or Sabauroud dextrose agar as the test medium and incubating cultures at 30°C until growth of the positive control covered approximately 90% of the agar plate.
The MIC was recorded as the lowest concentration of test material where at least five out of the six readings showed no growth. Growth of less than 5 mm around the inoculums was considered a negative result. Growth of greater than 5 mm around the lawn was considered a positive result.
Results
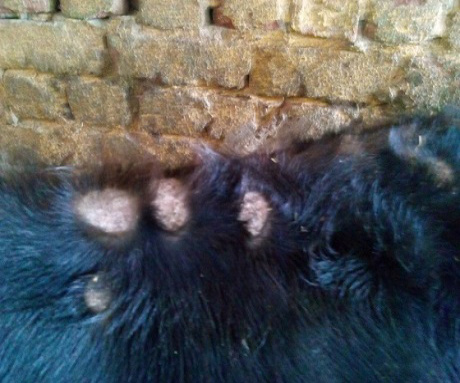
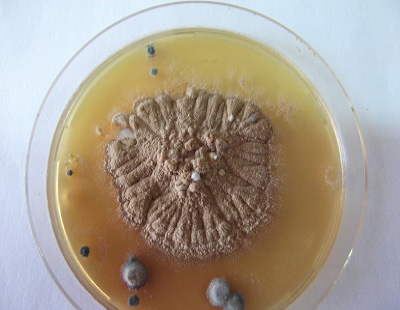
The study was carried out during September 2013 till April 2014 (Autumn and Winter months) in a farm containing 250 beef calves (local mixed breeds) aged 6-9 months. Clinical signs of skin lesions appeared on 30 animals after 2 weeks of receiving calves in the form of Circumscribed areas of hair loss filled with raised white scales on head, neck or all over the body (Figure 1, 2 and 3) also skin lesions also appeared on hands and face of 2 workers in the farm in contact with diseased animals. All examined samples were positive for fungal elements (ectothrix spores and endothrix hyphae) by direct microscopic examination. The fungus grew slowly on SDA producing white, cottony, heaped and slightly folded colonies with some submerge growth and yellow reverse pigment (Figure 4). Microscopic examination of isolates stained with lactophenol cotton blue revealed septate hyphae with numerous clavate microconidia borne laterally from the hyphae and many clamydospores arranged in chains (chains of pearls) characteristic of T. verrucosum.
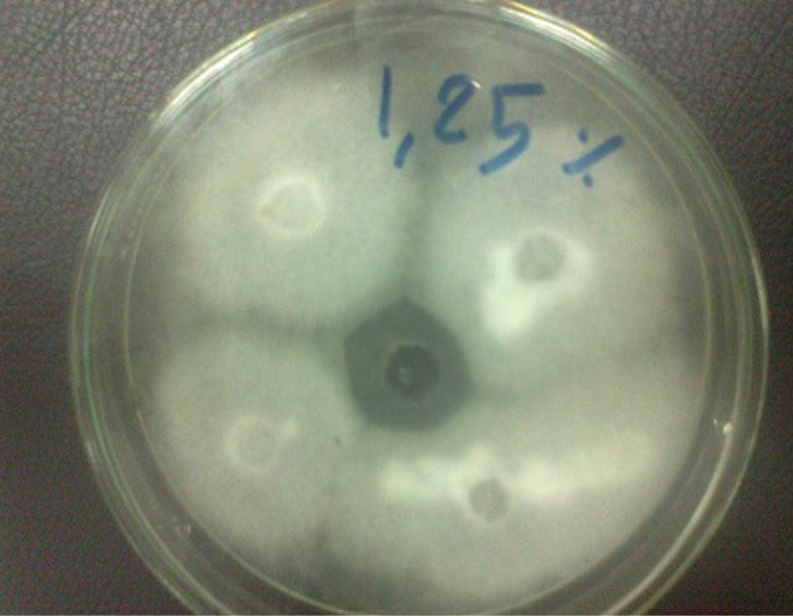
Figure 5: Trichophyton verrucosum grow on potato dextrose agar and shown antifungal activity bergamot 1.25% with zone of inhibition 28 mm

Figure 6: Trichophyton verrucosum grow on potato dextrose agar and shown antifungal activity bergamot 2.5% with zone of inhibition 32 mm

Figure 7: Trichophyton verrucosum grow on potato dextrose agar and shown antifungal activity bergamot 5% with zone of inhibition 40 mm
Antimicrobial Activity of Bergamot oil
The Bergamot oil has antifungal activity against a Trichophyton verrucosum in oil concentration 1.25%, 2.5% and 5% as a zone of inhibition of 28mm, 32mm and 40 mm in figure 5, 6 and 7, respectively.
Disscussion
Dermatophytosis is one of the economically important fungal disease of animals and has a zoonotic importance. The study was carried out in a beef farm at Giza governorate during the period between September 2013 and April 2014. After 2 weeks of receiving the calves aged 6-9 months skin lesions appeared on 30 calves out of 250 (12%). Lesions appeared in the form of circumscribed area of alopecia filled with white scales on face, head neck or all over the body. Trichophyton verrucosum was the only fungus isolated from affected the examined calves. This indicates that T. verrucosum is the main fungus causing ring worm in cattle as reported by Dalis et al., 2014. The dermatophytosis may be a major problem in calves specially under stress conditions like transportation and changing the place and managemental conditions as in the farm under study and that agree with previous findings of Dalis et al. (2014); Cam et al. (2007); Shams-Gahfarokhi et al. (2009) that young animals are particularly susceptible to infection by ringworm
fungi. This could be as a result of the poorly developed immune system and the high pH of the skin in young animals (Radostits et al., 2007). Diagnosis of ringworm in this study was based on clinical signs, demonstration of fungal elements in samples by direct microscopic examination and the isolation of causative agent by culture. T. verrucosum had been implicated in ringworm of cattle (Dalis et al. (2014); Swai and Sanka, 2012; Cam et al., 2007). The main clinical signs observed among the affected calves were circular, circumscribed, grayish-white, thick crusty lesions perceptibly raised above the skin. The lesions were most frequently found on the head and neck especially around the eyes and face. These observations were in agreement with other reports of Dalis et al. (2014); Akbarmehr (2011); Cam et al. (2007). The reason for the occurrence of more lesions around the eyes and face in young animals is not well understood. However, the habit of licking and grooming by calves could predispose this part of the animal to infection. Our results showed that all the samples examined by direct microscopy were positive for fungi. Other researchers found out that direct microscopic examination could provide a positive diagnosis in 60-71% of samples from which dermatophytes were isolated as in case of Dalis et al. (2014); Al-Ani et al. (2002); Sparkes et al. (1993). In this study, the dermatophyte was isolated in pure culture suggesting that SDA is a suitable selective medium for the isolation of pathogenic fungi. Colonies of T. verrucosum in this report were slow growing, white, cottony, heaped and slightly folded with some submerged growth and yellow reverse pigment. This observation is consistent with the findings of Dalis et al. (2014) and Forbes et al. (2002).
The warm and humid climate of our environment could have favoured the growth and development of fungal spores thereby predisposing the animals to infection and hence the outbreak in this highly susceptible population. Cattle ringworm causes high economic losses especially in the livestock and leather industries due to downgrading of hides and skin and decrease in meat and milk production vv as mentioned by Gudding and Lund (1995). The major problem with T. verrucosum infection in cattle farms is that, once the disease is introduced into a farm, it spreads rapidly among susceptible animals. The organism is difficult to eradicate from the environment because of the peculiarity in composition of its spores.
Treatment of cattle ringworm is expensive and cumbersome especially on a herd level as mentioned by Gudding and Lund (1995), this may be due to difficult application of most antifungal drugs also they are expensive. In this study we evaluate the antifungal activity of Bergamot oil (Citrus Bergamia) against Trichophyton verrucosum in order to provide an effective cheap antifungal drug easy for application. In the study we use different concentrations of Bergamot oil 1.25%, 2.5% and 5% and use it alone and in combination with salicylic acid. The study reported that Bergamot oil has antifungal activity against Trichophyton verrucosum at all concentrations (1.25%, 2.5% and 5%) with and without combination with salicylic acid. The study results give substantial support to popular or anecdotal beliefs in the effectiveness of treating skin with Bergamot oils. Also, the Antimycotic action of Bergamot oil especially on Trichophyton verrucosum and we recommended the application in vivo and determine the antifungal activity and the best method for application.
Conclusions
In the present study we could conclude that, Trichophyton verrucosum is the main cause of ring worm in cattle and has an occupational hazard appeared mainly in young calves under stress conditions. The study also high lights the significant antifungal properties of bergamot oil.
Consequently, in the field of elimination of ringworm infection, bergamot oil could be proper candidates disinfectant agents and could be used as active ingredient for dermatological applications.
ACKNOWLEDGEMENTS
We would like to thank Dr Shehab El Din Talat at NODCAR for his assistance in editing this research.
References