Advances in Animal and Veterinary Sciences
Research Article
Bovine Brucellosis in Organized Farms of India - An Assessment of Diagnostic Assays and Risk Factors
Rajeswari Shome1, B Shankaranarayan Padmashree1, Natesan Krithiga1, Kalleshamurthy Triveni1, Swati Sahay1, Bibek Ranjan Shome1, Padma Singh2, Habibur Rahman1
1National Institute of Veterinary Epidemiology and Disease Informatics (NIVEDI) (Formerly PD_ADMAS), Hebbal, Bangalore- 560 024, India; 2Department of Biotechnology, 6th Floor, Block-II, CGO Complex, Lodhi Road, New Delhi – 110 003, India.
Abstract | Bovine brucellosis is a highly contagious ubiquitous reproductive disorder of dairy animals. To assess the diagnostic assays and risk factors associated with bovine brucellosis, a systematic study was conducted in 24 organized farms, containing a total of 1359 dairy animals during the period of 2013-2014. Herd and animal level data were recorded in a structured questionnaire. Of the 1359 samples, 71 (5.22%), 82 (6.03%), 73 (5.37%) and 54 (3.97%) samples were positive by RBPT, iELISA, serum and blood PCRs respectively. Combination of iELISA and serum PCR were found most suitable to declare brucellosis status of the animals. The high brucellosis prevalence was recorded in medium sized farms (26-100 animals) than in small and large farms. Similarly, disease prevalence ranged from 6.13 to 11.42% in the age groups of 2 to 8 yrs. The disease predisposition with respect to breeds revealed that the prevalence varied from 3 to 5% in cross breeds of Holstein Friesian and Jersey, and serological and PCR tests were negative in Indian breeds such as Hallikar and Ongole cattle and Surti buffaloes. Purchase of animals without prior diagnosis, lack of awareness and routine milk testing were found as other potential risk factors for transmission of disease. The study facilitates improvisation of sensitivity of clinical surveillance system for early diagnosis and to prevent the disease transmission.
Keywords | RBPT, iELISA, PCR, Bovine brucellosis, Risk factors
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 04, 2014; Revised | October 24, 2014; Accepted | October 25, 2014; Published | November 06, 2014
*Correspondence | Rajeswari Shome, National Institute of Veterinary Epidemiology and Disease Informatics (NIVEDI) (Formerly PD_ADMAS), India; Email: rajeswarishome@gmail.com
Citation | Shome R, Padmashree BS, Krithiga N, Triveni K, Sahay S, Shome BR, Singh P, Rahman H (2014). Bovine Brucellosis in organized farms of India - An assessment of diagnostic assays and risk factors. Adv. Anim. Vet. Sci. 2 (10): 557-564.
DOI | http://dx.doi.org/10.14737/journal.aavs/2014/2.10.557.564
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2014 Shome et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Currently, Indian dairy is one of the world’s rapidly growing livestock industries, stands first in the world in milk production and accounts for more than 15% of world’s total milk production (Kumar and Prabhakar, 2013). Despite being the largest milk producer in the world, there is still great demand for milk and dairy products. Growth of dairy industry effectively depends on productive and reproductive performance of the healthy dairy animals, which in turn depends on prevalent bovine diseases of the country.
Brucellosis is one of the highly contagious ubiquitous reproductive diseases of dairy animals and highly prevalent among bovine population of the country (Patel et al., 2014) thus leading to an annual economic loses to the tune of US$ 58.8 million (Kollannur et al., 2007). The economic losses is because of abortions, stillbirths, reduced milk production, infertility (McDermott and Arimi, 2002) and revenue losses due to international trade impediment for animals and their products (Corbe, 2006). Brucellosis is also the second most important zoonotic disease of the world after rabies (FAO, 2005). Since more than three-fourth of population of rural India is in direct contact with bovine population which provides greater probability of zoonotic transmission of infection from animals to humans (Mantur and Amarnath, 2008). Thus effective control and eradication of bovine brucellosis is a global concern and can be achieved only by early, reliable and accurate diagnosis and vaccination. But brucellosis is a complicated disease in terms of diagnosis because of non-pathognomonic nature of infection and the clinical diagnosis cannot be generalized to all age groups, sex, breed and physiological status especially in non-pregnant animals, heifers and bulls. As a result, many cases remain under diagnosed and cause outbreaks in organized dairy farms, there by spreading the disease to other animals and humans (Bronner et al., 2014). This strikingly demands detailed study to assess the diagnostic tests and risk factors for brucellosis in organized farms with respect to species, age, sex, breed, herd size and associated risk factors. The information will be useful for improvisation of sensitivity of clinical surveillance system for early diagnosis and to prevent the disease transmission.
MATERIALS AND METHODS
Sample Outlay
A total of 1359 bovine samples [cattle (n=1270) and buffalo (n= 89)] were collected from 24 different organized farms (comprising of 100, 690 and 569 animals from small, medium and large dairy farms) in six districts of Karnataka (Bangalore Rural, Kolar, Chikabalapur, Mandya, Ramnagar and Mysore) by purposive sample approach based on farmers request. All the samples were used to evaluate the disease prevalence by various tests. Herd and animal level data were recorded in a structured questionnaire comprising of age, sex, breed, farm size, breeding methods used [natural or artificial insemination (AI)], history of abortion, routine brucellosis testing in the farms, purchased or raised animals in the farms, S.19 vaccination and brucellosis awareness level of the farmer. The categorization of farms is based on number of animals, less than 25 animals as small, 26-100 as medium and more than 100 animals as large farms (Chand and Chhabra , 2013). Of 1359 animals, 31 (2.28%) and 1328 (97.71 %) were males and females respectively and belonged to different age groups from 1 to 12 years.
The samples (both blood and serum) were collected from various cross breeds of Holstein Friesian (HF) and Jersey (J), Indian cattle breeds (Hallikar and Ongole), buffalo breeds (Surti and Murrah). Approximately 10mL of blood sample was collected from the jugular vein of each animal using vacutainers with and without EDTA (Becton Dickson, UK). Samples were labelled using codes describing the specific animal and herd. The clotted blood in the tubes were centrifuged at 3000g for 20 minutes to obtain clear serum and stored at −20°C until tested. The blood with EDTA was stored at 4°C for extraction of DNA.
Serological Tests
RBPT
All the serum (n=1359) samples were analysed by rose bengal plate test (RBPT) according to standard protocol (Alton et al. 1988). The B. abortus S99 colored antigen was procured from Institute of Animal Health and Veterinary Biologicals (IAH&VB), Hebbal, Bangalore, India.
Protein G Based Indirect Enzyme Linked Immunosorbent Assay (iELISA)
For the assay, smooth Lipopolysaccharide (sLPS) antigen from standard strain B. abortus S99 was extracted as per the OIE protocol (OIE, 2011). The polysorp microtiter plates (Nunc, Germany) were coated with 1:300 dilution of sLPS antigen at 100μl per well (10ng/well) in carbonate-bicarbonate buffer (pH 9.6) and incubated at 4°C for overnight. Antigen coated plates were washed three times with PBST wash buffer pH 7.2 (Phosphate buffered saline containing 0.05 % Tween 20). Test and control sera diluted in PBST blocking buffer (1:100) containing 2% bovine gelatine was added to respective wells (100μl) of the plates in duplicates (test sera) and quadruplicate (control sera) and incubated at 37°C for 1hour. The plates were then washed as mentioned earlier. The recombinant protein-G horse radish peroxidase (HRP) conjugate (Pierce, Germany), diluted 1:8000 in PBST buffer were added to all the wells (100μl) and incubated for 1hour at 37°C on orbital shaker (300r.p.m./min). After washing, freshly prepared o-Phenylenediamine dihydrochloride (OPD), (Sigma, Germany) solution containing 5mg OPD tablet in 12.5 ml of distilled water and 50μl of 3% H2O2 was added and kept for colour development for 10 minutes. Enzyme-substrate reaction was stopped by adding 1M H2SO4 (50μl) and colour development in the form of the optical density (OD) was read at 492 nm using an ELISA microplate reader (Bio-Rad). Percent positivity (PP) values which were used for the diagnostic interpretations were calculated as follows:
Percent positivity (PP)= (Average OD value of test serum)/(Median OD value of strong positive control) x 100
The cut-off values established for diagnosis was decided after thorough screening and validation (Shome et al. 2011). Any sample of PP value below 55% is taken as negative, between 55-65% as moderate positive, more than 65% as strong positive and sample with only 55% PP are recommended for re-sampling for confirmation.
Brucella Genus Specific Serum and Blood PCRs
The genomic DNA from serum and blood samples was extracted using DNA easy blood and tissue kit (QiAgen, USA). For detection of Brucella genus specific sequences by PCR, genus primers were used (Baily et al., 1992). The PCR reaction was carried out in 25μl reaction mixture containing of 12.5μl of 2x PCR-master-mix [0.5units/μl Taq DNA polymerase in reaction buffer, 4 mM MgCl2, 0.4mM dNTP (Fermentas)], 5pmol each of forward and reverse primers and 50ng of DNA template. The DNA amplification reaction was performed in a Master Cycler Gradient Thermo cycler (Eppendorf) with a preheated lid. The resultant PCR products were analysed by 1.5% agarose gel electrophoresis stained with ethidium bromide.
To rule out false negative and false positive results, second sampling has been carried out after 45 days of first collection and all the samples were retested by all the tests. Positive disease status was confirmed based on results of paired samples by at least by two different tests (Nielsen et al., 2006; OIE, 2011).
Statistical Analysis
By using chi square test, significance of difference was determined and value of p< 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software version: 22 (IBM, India).
RESULTS AND DISCUSSION
Analysis of Prevalence Status
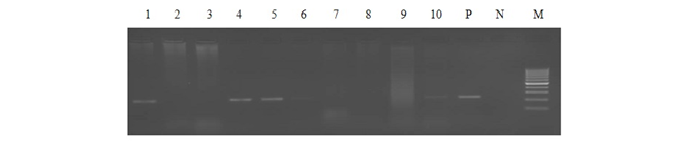
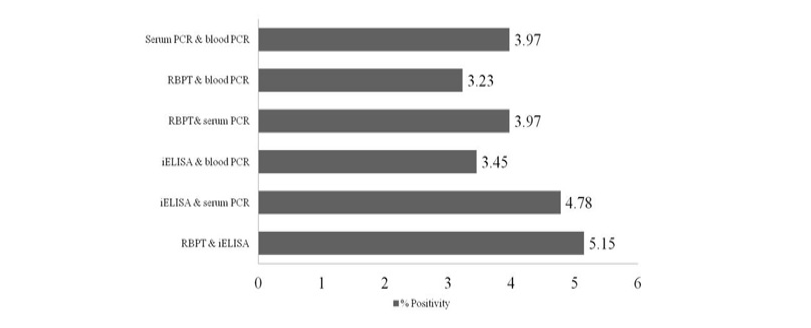
Out of 1359 serum samples, 71 (5.22%) and 82 (6.03%) were detected positive by RBPT and iELISA, respectively. Similarly, 73 (5.37%) and 54 (3.97%) serum and blood DNA could amplify 223bp product in PCR, respectively (1 and Figure 1). Comparative evaluation of tests revealed that 44 (3.23%) samples were positive by all the four tests and 17 (1.25%) seropositive cases were negative by PCRs. Similarly, 07 (0.51%) seronegative cases were solely detected by serum and blood PCRs. Due to the absence of gold standard isolation and culture, paired samples positive by at least by any two tests were considered to calculate prevalence of disease. Overall prevalence was found to be 6.62% (90/1359) and combined percentage positivity of different diagnostic tests is depicted in table 2 and figure 2.
Comparison of both the serological tests (RBPT and iELISA) revealed the superiority of iELISA over RBPT as it could detect more number of samples than RBPT. Also iELISA could detect more number of samples compare to all other tests performed in the study (6.03%). Though RBPT is the definite test for Brucella screening in many countries; but the test has several limitations (Munoz et al., 2005; Poester et al., 2010).
The persistence of IgG antibody for longer period in recovered or vaccinated animals are being detected by the high sensitivity of iELISA. Hence, the seroprevalence by iELISA could reflect either past or present exposure to Brucella organisms. Since brucellosis vaccination has not done in the farms investigated, the vaccinial antibody is ruled out. The seropositivity by iELISA in the current study could be either due to active/ chronic/ recovered status of the animals. Hence, it is always recommended that the combination of iELISA with antigen detection test should be used to minimize the false reactions.
Lane 1-5: bovine serum DNA samples, Lane 6-10: bovine blood DNA samples, Lane P: positive control (B.abortus S99), Lane N: no template control and Lane M: 100bp ladder.
PCR using serum and blood DNA showed that the serum PCR has better sensitivity over the blood PCR. Out of 5.15% of seropositive samples, higher caseswere picked up by serum PCR (5.37%) in comparison to blood PCR (3.97%). The study of Zerva et al. (2001) also reported serum samples to be the preferred clinical specimen over whole blood for the molecular diagnosis of brucellosis. Whole blood and serum samples are the easiest samples to use in terms of collection, processing and pose lower risks to personnel in the laboratories. Moreover, serum lack most potent inhibitors of Taq polymerase and highly suitable for long term storage. False-negative reactions in PCR can occur as a result of presence of EDTA, RNase or DNase, heme, heparin, phenol and other reagents. Till today, the problem of absolute diagnosis of bovine brucellosis remains unanswered and possible reason could be the latent infection. This is also evident from the current study that the 17 (1.25%) seropositive cases were negative by both the PCRs (Figure 2).
Analysis of Risk Factors
Five risk factors associated viz., species, sex, age, herd size and breed in relation to prevalence of bovine brucellosis were analysed and tabulated in table 3. All the farmers were interviewed from 24 herds resulting in a 100% response rate for participation in the study. The species wise prevalence showed that brucellosis is highly prevalent in buffaloes (39.32%) than cows (4.33%). This may be due to very high prevalence recorded in one of the buffalo farm and also the small sample size of buffaloes compared to the cows in this study. The higher disease prevalence in buffaloes in comparison to cattle has been reported by other researchers (Dhand et al., 2005; Chand and Chhabra, 2013). Thus, for any control program to be successful, both species will have to be included.
Table 1: Test wise prevalence of bovine brucellosis in organized farm
|
RBPT +ve |
iELISA +ve |
Serum PCR +ve |
Blood PCR +ve |
Seropositive & PCR -ve |
Seronegative & PCR +ve |
Positive by all four tests |
Positive by any two of the four tests |
|
71 (5.22)* |
82 (6.03) |
73 (5.37) |
54 (3.97) |
17 (1.25) |
07 (0.51) |
44 (3.23) |
90 (6.62) |
*values in parenthesis represent percentage
Table 2: Comparative analysis of tests for diagnosis of bovine brucellosis in organized farms
|
RBPT |
Serum PCR |
Blood PCR |
||||
Negative (1288) |
Positive (71) |
Negative (1286) |
Positive (73) |
Negative (1305) |
Positive (54) |
||
ELISA |
Negative (1277) * Positive (82) |
1276 (93.8) |
01 (0.07) |
1269 (93.37) |
08 (0.58) |
1270 (93.45) |
7 (0.51) |
12(0.88) |
70(5.15) |
17(1.25) |
65(4.78) |
35(2.57) |
47(3.45) |
||
RBPT |
Negative (1288) Positive (71) |
1269 (93.37) |
19 (1.39) |
1278 (94.04) |
10 (0.73) |
||
17 (1.25) |
54 (3.97) |
27 (1.98) |
44 (3.23) |
||||
Serum PCR |
Negative (1286) Positive (73) |
1286 (94.62) |
0 |
||||
19 (1.39) |
54 (3.97) |
||||||
*values in parenthesis represent percentage
Sex-wise prevalence revealed that, 2 out of 31 males and 88(6.62%) out of 1328 females were positive and statistically insignificant (P value- 0.969). However, testing for brucellosis in male animals used for semen collection every six months is made mandatory in the country to control the spread of brucellosis through AI. The low prevalence in males is in agreement with that of other workers (Kubuafor et al., 2000). Seroprevalence up to 6.63% is revealed in females which may be due to lack of periodical screenings in large female bovine population (unlike meager breeding male population) and also undiagnosed infected females causes increased spreading of the disease in the herds.
Disease prevalence by all the tests was found high (12.6%) in the medium size herd whereas low in large herds (0.52%). The highest prevalence in medium size farms can be explained either due to indiscriminate replacements of animals from herds of unknown brucellosis status and poor hygiene. However, it is well known fact that brucellosis is commonly spreads through increased direct or indirect contact via feed especially following abortions and promoting transmission of Brucella organism (McDermott and Arimi, 2002). The greater chances of spreading of infection have been found especially in organized herds than in marginal herds (Jain et al., 2013). The low prevalence in large sized farms indicates good farm management practices, periodical milk ring testing and care of the animals by trained personnel. Similarly brucellosis positivity has not been recorded in small farms which may be attributed to various factors like sufficient unit floor space for each animal; stall feeding that minimizes contact with other infected animals and being small size farm, possibly more personnel attention to the animals by the farmer himself.
Table 3: Risk factors associated with general characteristics of bovines in transmission of bovine brucellosis
|
Risk Factors |
No. of animals |
No. of Positives |
No. of Negatives |
χ2 |
P-value* |
|
|
Species |
Cow Buffalo |
1270(93.45) 89 (6.54) |
55 (4.33) 35 (39.32) |
1215 (95.66) 54 (60.67) |
164.71 df-1 |
<0.001* |
|
Sex |
Male Female |
31 (2.28) 1328 (97.71) |
02 (6.45) 88 (6.63) |
29 (93.5) 1240 (93.37) |
0.0015 df-1 |
0.969 |
|
Size of the herd |
Small (< 25 animas) Medium (26-100 animals) Large (>100 animals) |
100 (7.35) 690 (50.77) 569 (41.86) |
0 87 (12.6) 03 (0.52) |
100 (100.00) 603 (87.39) 566 (99.47) |
81.26 df-2 |
<0.001* |
|
Age (yrs) |
1-2 2.1-3 3.1-4 4.1-6 6.1-8 Above 8 |
253 (18.61) 701 (51.58) 218 (16.04) 148 (10.89) 35 (2.57) 4 (0.29) |
08 (3.16) 43 (6.13) 19 (8.71) 16 (10.81) 4 (11.42) 0 |
245 (96.8) 658 (93.86) 199 (91.28) 132 (89.18) 31 (88.57) 4 (100.00) |
12.50 df- 5 |
0.029* |
|
Breed |
Holstein Frieian (HF) cross Jersey (J) cross Murrah Surti Ongole Hallikar Bulls |
799 (58.79) 375 (27.59) 64 (4.70) 25 (1.83) 62 (4.56) 03 (0.22) 31 (2.28) |
40 (5.00) 13 (3.46) 35 (54.6) 0 0 0 2 (6.45) |
759 (94.99) 362 (96.53) 29 (45.31) 25 (100.0) 62 (100.00 3 (100.0) 29 (93.5) |
8.19 8.34 251 1.81 4.61 0.0213 0.0015 |
0.004* 0.0038* <0.001* 0.179 0.032* 0.884 0.969 |
* P- value <0.05 is considered as significant
Table 4: Risk factors associated with farm management in transmission of bovine brucellosis in organized farms
|
Risk Factor |
No. of Farms |
No. of infected farms |
Herd prevalence (%) |
χ2 |
P-value |
No. of Animals |
No. of infected animals |
Individual animal prevalence (%) |
χ2 |
P-value |
|
|
Breeding method |
Natural AI |
03 21 |
2 09 |
66.67 42.85 |
0.599 df-1 |
0.439 |
114 1245 |
23 67 |
20.17 5.38 |
36.962 df-1 |
<0.001* |
|
History of abortion & repeat breeding |
Yes No |
11 13 |
08 03 |
72.73 23.08 |
5.916 df-1 |
0.015* |
949 410 |
84 06 |
8.85 1.46 |
25.271 |
<0.001* |
|
Purchase of Animal |
Yes No |
11 13 |
08 03 |
72.73 23.08 |
5.916 df-1 |
0.015* |
1032 327 |
80 10 |
7.75 3.05 |
8.847 df-1 |
0.003* |
|
Routine milk diagnosis |
Yes No |
11 13 |
02 09 |
18.19 69.23 |
6.254 df-1 |
0.012* |
388 971 |
03 87 |
0.77 8.95 |
30.045 df-1 |
<0.001* |
|
Brucellosis awareness |
Yes No |
12 12 |
02 09 |
16.67 75.00 |
8.224 df-1 |
0.004* |
517 842 |
03 87 |
0.58 10.33 |
49.264 df-1 |
<0.001* |
* P- value <0.05 is considered as significant
Age wise prevalence of disease showed low in young (1-2yrs) and older age group of animals (≥ 8 yrs) than in the age groups of 2 to 8 yrs (Table 3). The highest percentage (51.58) of animals had an average age of 2.1-3years which constitutes about half of all the age groups in the study. The susceptibility to disease increases with age and more commonly associated with sexual maturity than age (Radostits et al., 2000). Few seropositives were detected in less than one year age group of animals and it may be due to exposure to brucellosis infected animals in the farms. It has been described for brucellosis that some of the infected animals, do not become seropositive until pregnant and younger animals are more resistant to primary infection and frequently clear infections, although latent infection do occur (Walker, 1999).
The disease predisposition with respect to breeds revealed that Indian breeds such as Hallikar and Ongole cattle and Surti buffalo breeds were negative by serological and PCR tests (with exception in Murrah buffaloes where 50% of the animals were positive by both antigen and antibody detection tests.). In cross breeds of HF and Jersey, the prevalence ranged from 3 to 5%. The breed wise prevalence of brucellosis is statistically significant is in good agreement with other researchers (Akbarmehr and Ghiyamirad, 2011; Khurana et al., 2012; Patel et al., 2014; Bakhtullah et al., 2014).
Association of farm management practices as risk factors for brucellosis were analysed and tabulated in table 4. Purchase of animals without prior screening, lack of awareness about the disease and absence of routine milk ring tests are figured out as major risk factors for the transmission of brucellosis at both herd and individual animal level. Purchase of animals is a major mode of acquisition of new infected animal among small and medium size farm holders. Hence prior screening for brucellosis before purchase has to be made mandatory to prevent introduction of positive reactors into new farms (Chand and Chhabra, 2013).
AI is routinely practiced in cattle and buffaloes and natural breeding is opted in indigenous cattle breeds (Ongole and Hallikar) in the country. Out of 24 farms investigated, in three farms, natural breeding was practiced. Statistically significant difference was observed among the two breeding practices for the transmission of brucellosis (P-value < 0.001) at animal level, but at herd level, there was no statistical significance. Screening of males regularly in semen collection centres and use of Brucella free semen are strictly being followed in the country. However, natural breeding is still practiced in some remote and tribal areas which need attention.
The study revealed that disease is more prevalent and statistically significant in those farms where abortion and repeat breeding problems are reported (Table 4). Hence prompt veterinary care and timely laboratory testing assistance should be utilized to diagnose causation of abortions, premature births, repeat breeding or other clinical signs. Careful selection of animals before purchase from Brucella-free herds, pre-purchase tests and quarantine needs to be followed to keep the herd free of brucellosis.
CONCLUSION
From present study it can be concluded that it is very difficult to detect all infected cases of bovine brucellosis using a single test. Hence, combination of both antigen and antibody detection tests are highly useful for declaring the status of the disease in the farms. The serum sample was found suitable clinical sample for PCR and serological testing for diagnosis of brucellosis. Considering high economic losses it can cause on livestock sector and possible human health hazard, timely and accurate diagnosis, facilitation of awareness generation program and adoption of proper prevention and control strategies are recommended.
CONFLICT OF INTEREST
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
ACKNOWLEDGMENT
This work is supported by the Department of Biotechnology, New Delhi, through the DBT-Network Project on Brucellosis, are gratefully acknowledged. We also acknowledge the support of office assistant Mr. H.M. Manu Kumar in sample collection and data analysis.
REFERENCES