Advances in Animal and Veterinary Sciences
Research Article
Studies on the Anthelmintic and Antioxidant Activity of Root Extracts of Elytraria Acaulis
Karthikeyan Jagadeesan, Thooyavan George,Mullai Nila Kamalanathan, Ilayaraja.T
Department of Zoology, Presidency College (Autonomous), Chennai – 600 005, India.
Abstract | Elytraria acaulis, a traditional plant used to cure worm infection has been evaluated for its anthelmintic and antioxidant activity. Adult parasites of Moniezia expansa collected from the gut of Ovis aries were exposed to 25, 50, 100 mg/ml concentration root extracts. Albendazole concentration of 10 mg/ml was taken as positive control, with negative control of PBS in 1%DMSO. Physical activity, concentration of carbohydrate and protein along with the light microscopic observations were taken as the parameters to assess the anthelmintic potential of Elytraria acaulis. The control group parasites incubated with 1% DMSO (Dimethyl Sulfoxide) survived for more than 58 hours. In vitro treated parasites showed a dose dependent activity on the onset of paralysis, with 100mg/ml concentration. Paralysis was observed after 32.95±0.095 and 48.40±0.141 minutes. With reference to methanol and ethanol extracts respectively, the parasites incubated with methanol extract, the death of the parasites were recorded after 45.05±0.122 min. The concentration of carbohydrate and total protein levels were marginally elevated after treatment. Deformation in the subtegument layer along with the vacuolization of parenchymal tissues was evident. The antioxidant activity of the methanol extract of the plant shows a free radical scavenging activity of 79.38% with 200 µg/ml concentration. Anthelmintic and antioxidant efficacy of the tested plant is because of the presence of various phytoconstituents. Methanolic root extract of Elytraria acaulis seems to be a potent agent for anthelmintic with antioxidant activity. Further studies may throw light on the active compounds present in the crude extracts.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Revised | September 01, 2014; Accepted | September 02, 2014; Published | September 27, 2014
*Correspondence | Karthikeyan Jagadeesan, Presidency College (Autonomous), Chennai, India; Email: j_karthikeyan@hotmail.com
Citation | Jagadeesan K, George T, Kamalanathan MN, Ilayaraja.T (2014). Studies on the Anthelmintic and Antioxidant Activity of Root Extracts of Elytraria Acaulis. Adv. Anim. Vet. Sci. 2 (8): 457-463.
DOI | http://dx.doi.org/10.14737/journal.aavs/2014/2.8.457.463
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2014 Jagadeesan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
IntroductioN
Parasitic cestode infections is a well-recognized problem around the globe (Perry and Randolph, 1999). From time immemorial cestode helminth parasites remained as a group of successful organisms living at the expense of their domestic sheep, goat and cattle hosts. Intestinal parasites could cause severe sabotage to sheep in terms of high mortality, reduced body weight, wool yield and birth rate (Ahmad,1992), severe illness or death of the host (Soulsby, 1986) Monieziasis is an important problem in sheep breeding (Becker et al.,1981, Polec, 1990). However, till date the control of cestodes were purely based on the use of chemical anthelmintics, repeated usage of these drugs resulted in the development of resistance to various families of chemical anthelmintics (Chartier et al., 2001; Wolstenholme et al., 2004; Papadopoulos, 2008). The search for alternative solution to chemical treatments is a priority to achieve a more sustainable control of the parasitism. In recent years there has been a great upsurge of interest in analysing the impact of parasites towards herbal anthelmintics. Plant/herbal based medicines have become indispensable and are forming an integral part of the primary health care system in many nations including India (WHO, 2002-05). Extracts of different parts of the plants have been used to combat worm infections in many parts of the world. Naturally occurring compounds in plant extracts have potency to kill or eliminate the parasites without harming the host (Nasser et al., 1997). Elytraria acaulis belongs to the family Acanthaceae locally called as Nilakadambai or Pumikatambu in Tamil is widely distributed, stem less perennial herb with unbranched flowering stem, commonly used to cure the worm infections in the wounds of cattle by the traditional healers. The present study aims to evaluate the anthelmintic efficacy of the root extracts of the plant with reference to biochemical and light microscopic observations along with the antioxidant activity for the effective treatment of parasites, thereby reducing the economic loss incurred due to infections.
Material and Methods
Collection of Animals
Small intestine of the sheep (Ovis aries) was collected from the slaughter house at Perambur, Chennai, India. The small intestines were opened in a wide container and washed with 0.9 % Phosphate buffered saline (PBS). The freshly obtained worms were identified and used for in vitro studies.
Extract preparation
Fresh roots of Elytraria acaulis were collected in the month of October and November from neighbouring villages of Villupuram (Tamil Nadu). The roots were washed thoroughly with water and cut into small pieces. The roots were shade dried and powdered. About 50grams of powder was soaked in 500ml of solvent before extraction using soxhlet apparatus. The solvents were concentrated using rotary vacuum evaporator, the crude extracts (7.88% w/w) were stored in refrigerator at 2°- 8° C for further usage.
Phytochemical Analysis
The root extracts were analysed for the presence of the following secondary metabolite phyto constituents such as tannins, saponin, flavonoids, alkaloids, quinones, glycosides, terpenoids, triterpenoids, phenols, cumarins, acids, cyanins, cardiac glycosides, proteins and carbohydrates using modified and standard protocols (Obasi et al., 2010; Tiwari et al., 2011).
Antioxidant Activity
The free radical scavenging activity of methanolic and ethanolic root extracts Elytraria acaulis was assessed using modified procedure (Khalaf et al., 2008) DPPH solution 0.004% was prepared in ethanol and 5mL of this solution was mixed with the same volume of extract (100, 200, 300, 400 µg/mL) and standard solutions separately. The solutions were kept in dark for 30minutes. If free radicals have been scavenged, DPPH purple colour will degenerate to yellow colour confirming the free radical scavenging activity. The absorbance of the mixture was taken at 517 nm using UV-Vis spectrophotometer (UV-10, Thermo). The scavenging activity of the extract against DPPH was calculated using the following equation:
Scavenging Activity = (Absorbance of control-Absorbance of
samples)/ (Absorbance of control) x 100The percentage of scavenging activity was compared with positive control.
In vitro Experiments
The collected fresh alive M. expansa worms were thoroughly washed with PBS (Phosphate Buffer Saline), further they were incubated at 37° C ± 1 °C with 25 mg, 50 mg, 100 mg/ml concentration of ethanol and methanol root extracts of E. acaulis in 0.9% phosphate buffer saline in 1% DMSO. Albendazole (10 mg/ml) was used as positive control and PBS in 1% DMSO as negative control. Five replicates were used for each concentration. The time taken for complete inactivation, paralysis and death of the parasites were recorded. Paralysis was noted when there was no movement in the worm except when the worms were shaken vigorously. Death of the worms were recorded ascertaining that worms were not shown any movement after vigorous shaking and further confirmed by introducing the worms into worm water(45-50 °C) for observing the movement. The paralyzed tapeworms were processed for biochemical and light microscopic observations along with the control.
Estimation of Metabolites
Biochemical parameters were studied in immature, mature and gravid proglottid regions of the parasites. The total protein content was determined following Lowry’s method (Lowry et al., 1951). The amount of total carbohydrates present in the Trichloro Acetic Acid (TCA) soluble tissue extracts were estimated by Oligo and polysaccharide sensitive Phenol-Sulphuric acid method of DuBois et al. (1956).
Table 1: In vitro Anthelmintic efficacy of the methanol and ethanol root extracts of the Elytraria acaulis on Moniezia expansa (in Minutes).
|
10mg/ml |
25mg/ml |
50mg/ml |
100mg/ml |
|||||
|
Paralysis |
Death |
Paralysis |
Death |
Paralysis |
Death |
Paralysis |
Death |
|
|
Methanol |
- |
- |
143.83± 1.194 |
163.50± 0.886 |
118.82± 2.919 |
153.00± 1.154 |
32.99± 0.095 |
45.05± 0.122 |
|
Ethanol |
- |
- |
136.50± 1.56 |
166.83± 1.650 |
109.83± 0.812 |
145.50± 1.746 |
48.40± 0.141 |
57.05± 0.1075 |
|
Albendazole |
92.25± 0.155 |
105.20± 0.159 |
- |
- |
- |
- |
- |
- |
Light Microscopy
The Paralyzed tapeworms were fixed in 10 % formalin for light microscopic observation. Delafield hematoxylin and Eosin method of Humason, 1967 was adopted. The deparaffinized and hydrated sections were stained with aqueous Delafield hematoxylin for 2-3 minutes; the stained slides were further counter stained with 0.1% Eosin in alcohol for 15 – 20 seconds. The DPX mounted slides were observed for the changes.
Table 2: Concentration of carbohydrate (mg/100mg wet tissue) in the proglottids of the Elytraria acaulis root extract treated Moniezia expansa.
|
Carbohydrate |
Control |
Methanol |
Ethanol |
|
Immature |
1.010±0.011 |
1.843*±0.059 |
2.016*±0.035 |
|
Mature |
0.669±0.017 |
0.810±0.016 |
1.037*±0.031 |
|
Gravid |
0.378±0.010 |
0.532±0.007 |
0.725**±0.016 |
Mean ± SE *P<0.05, **P<0.01
Statistics
Mean ± Standard error (SE) were calculated for each sample group. To understand the level of significance between control and experimental animals, student’s t-test was applied. Chi-square values were calculated using Microsoft Excel of Windows 2007 for performing calculations. The calculations resulted in P <0.05 were considered to be statistically significant.
RESULTS
Different crude extracts of Elytraria acaulis were tested against Moniezia expansa to find the anthelmintic efficacy of the root extract. The results presented in Table 1 reveals a dose dependent effect over the parasite and it is evident from the time taken for the onset of paralysis and death of the organism. The time taken for paralysis with reference to treatment with methanolic and ethanolic root extracts (100mg/ml) were 32.99±0.09 min and 48.40 ± 0.141 min, respectively. However, the death was recorded at 45.05 ± 0.122 min and 57.05 ± 0.107 min after incubation with methanol and ethanol extracts respectively. In albendazole treated (10mg/ml) parasites the onset of paralysis was recorded after 92.25 ± 0.15 min followed by death of the parasite after 105.20 ± 0.15min.
Anthelmintic efficacy of the extract varies with reference to the concentration of different secondary metabolites present in the root extract of Elytraria acaulis. The methanolic and ethanolic root extracts were found to contain tannins, saponins, flavonoids, alkaloids, quanins, cumarins, cyanins and cardiac glycosides. Glucose is a very important energy source for many helminthes inhabiting the gut of vertebrates. The total glucose present in the different regions viz., immature, mature and gravid proglottids of Moniezia expansa are given in table 2. The total carbohydrates in the cestode parasites show an anteroposterior gradient in decreasing trend from immature, mature and gravid proglottids. In extract treated groups an increasing trend was observed. However, the percentage of increase was less in gravid segment when compared to mature and immature segments. In mature segment, carbohydrate level increases from 0.069 ± 0.017 to 0.810 ± 0.016 mg/100mg wet tissue in methanolic extract treated group and 1.037 ± 0.03 mg/100mg wet tissue in ethanolic extract treated group. In the immature segments the percentage of increase was significant (p<0.05) of about 82.47% and 99.60% respectively. The cestode lacks the gut, hence the absorption of nutrients takes place through the teguments. The amount of protein shows an anteroposterior gradient of increasing trend from immature to gravid regions of the parasites. Results given in table 3 reveals that in extract treated group total protein content increases significantly (p<0.01), to about 79.12% and 78.79% in the gravid segment of the parasite.
Table 3: Concentration of protein (mg/100mg wet tissue) in the proglottids of the Elytraria acaulis root extract treated Moniezia expansa.
|
Protein |
Control |
Methanol |
Ethanol |
|
Immature |
12.838 ± 0.0135 |
13.836 ± 0.03125 |
13.880 ± 0.0177 |
|
Mature |
14.433 ± 0.0171 |
19.033* ± 0.0339 |
19.260* ± 0.0383 |
|
Gravid |
15.330 ± 0.0145 |
27.460** ± 0.0181 |
27.49** ± 0.0628 |
Mean ± SE *P<0.005, **P<0.01
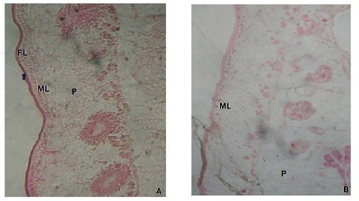
Light microscopic observations of control mature proglottid segment of Moniezia expansa reveals the outer epithelium has two distinct zones viz., tegument and subtegument layer along with the parenchymal cells and fibrous material occupying most of the space (Figure 1A and 1B). The area behind the subtegumentary layer is occupied by the gland cells along with parenchymal cells. The cross section of the parasites treated with the methanolic root extract of Elytraria acaulis clearly indicates the overall deformation of the tegumental layer, necrosis of inner subtegumental layer, vacuolization along with disorganized paranchymal tissues.
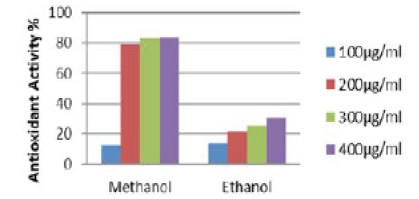
Quantitative analysis of antioxidant activity of methanolic and ethanolic extracts of the root of Elytraria acaulis (Figure 2) reveals tremendous variation in the antioxidant activity of scavenging DPPH (free radical) and converting into DPPHH. The methanol extract shows a concentration dependent radical scavenging activity. In methanolic root extract of Elytraria acaulis a high free radical scavenging activity of 79.38%, 82.92% and 83.5% in relation to concentrations of 200,300 and 400 µg/ml respectively. However, no drastic change in the free radical scavenging activity was observed with reference to the ethanolic extract where the percentage of scavenging activity does not exceed 30.05% with 400µg/ml concentration.
A: Photomicrograph of M.expansa showing tegument (T) Fibrous Layer (FL) Muscular Layer (ML) and Parenchyma Cells (P). B: Photomicrograph of methanolic extract treated M.expansa showing deformation of subtegumental layer and vacuolization of parenchymal cells.
DISCUSSION
Moniezia expansa a most prevalent helminth parasite plays an important role in the economic losses of farm animals (Bashtar et al., 2011). Anthelmintic substances of synthetic drugs were reported as a threat for human health (Cabaret, 2008). Secondary metabolites of plant derived extracts are responsible for anthelmintic activity (Aswar et al., 2008). Phytochemicals such as steroids, carbohydrates, tannins, triterpenoids, flavonoids and cumarines were responsible for the potent anthelmintic activity (Hossain et al., 2010). The present investigation reveals that anthelmintic activity of methanol root extract with the concentration of 100 mg/ml requires only 32.99 min for the occurrence of paralysis in treated parasites. The function of the synthetic anthelmintic drug is known to cause paralysis of the worm that are expelled in the faeces of the man and animals, the treatment with the root extracts cause the death of the parasite.
Natural substances with the anthelmintic activity is the need of the hour, since the molecules currently used to control helminthes are group restricted and expensive (Mehlhorn et al., 2010). The Elytraria acaulis, a traditional plant which is used to cure worm infection is one such plant with potent anthelmintic activity. Transcuticular diffusion is a common means of entry for non-nutrient and non-electrolyte substances in parasites. The major role played by the phytoconstituents is the alterations in the membrane permeability, a possible mechanism of action is that the constituents binds the glycoprotein of the cuticle of the parasite (Kumar et al., 2011; Thompson and Geary, 1995) there by causing death. Saponins are known to cause damages in the membrane of the parasite and causing vacuolization and disintegration of monogenea tegument (Wang et al., 2010). Moreover, the alkaloids has the ability to intercalate with protein synthesis of the parasites (Al-Shaibani et al., 2009). The results of the present investigation reveals that the anthelmintic efficacy of the tested plant is because of the presence of various phytoconstituents with varying functions. Better therapeutic effects of plant extracts may be obtained from a combination of active principles in each plant instead of single isolated compounds (Cho et al., 2003).
Glucose/free sugars and glycogen in the cestode parasites show an anteroposterior gradient of decreasing trend from immature, mature and gravid proglottids (Chopra, 1991). The same trend has been observed in the present investigation. Development and growth oriented changes in the total carbohydrates in segmented cestodes have been demonstrated (Bennett et al., 1990; Uglem and Pappas, 1991). It is evident from the results an anteroposterior gradient in the level of glucose concentration was observed in the parasites. Glucose and other simple carbohydrates are the main energy source for the cestodes and trematodes (Roberts, 1983). The immature region with higher carbohydrates represents the active region to utilize the energy source for proglottization process, whereas the mature segment with less amount of glucose probably accounted to their tissue differentiation especially the gonads. Energy derivation pathways involving carbohydrates and proteins hampered in the Achyranthes aspera treated cestode parasites (Prabhakaran, 2013). Synthetic phenolic compounds interfere with energy generation in the cestode parasites by uncoupling the oxidative phosphorylation, consequently leading to depletion in the production of ATP by the parasites. Increasing trend in the amount of total glucose in all the three regions of the tapeworm treated with methanol and ethanol extracts suggests that the breakdown of glycogen in treated group was found to be higher when compared to controls, this rapid glycogenolysis in the treated group may be the source for enhanced glucose level in all three regions of the parasite. The enhanced glycolytic activity witnessed in the treated parasites seems to be the function of anthelmintic stress caused by the test phytochemicals (Das et al., 2004). Protein occurs in different forms in parasites, the present study reveals the anteroposterior gradient of increasing trend in the total protein content, a step wise ascendency in the value of total protein content in immature, mature and gravid regions of M. expansa were observed (Rani et al., 1987). The immature segment is the metabolically most active region (Smyth, 1969). Our findings reveals that the non-utilization and accumulation of protein in the segments may be due to the imbalance between the parasite and host tissue. Further the phytoconstituents exerts an upset in the milieu interior and arresting the macromolecular synthesis.
Antioxidants act as a defence mechanism that protects against oxidative damage, and include compounds to remove or repair damaged molecules. It can prevent /retard the oxidation caused by free radicals and sufficient intake of antioxidants is supposed to protect against diseases. (Celiktar et al., 2007) Plant phenolics have drawn increasing attention due to their potent antioxidant properties and their marked effects in the prevention of various oxidative stress associated diseases such as cancer (Jin and Russell, 2010). Plant-phenols, flavonoids and tannins and terpenoids reported to possess antioxidant activity (Sanchez-Moreno, 2002; Maryam et al., 2009; Dragland et al., 2003). There was a close correlation between presence of phytoconstituents and the antioxidant potential. Methanolic root extract of Elytraria acaulis has a potential scavenging activity when compared to ethanolic root extract due to the absence of terpenoids and meager amount of the presence of flavonoids in the extract.
In conclusion Elytraria acaulis root extracts has the potential anthelmintic and antioxidant efficacy with reference to methanolic extract while the ethanolic extracts lack the antioxidant activity. Further studies will throw light to identify and evaluate the active components and their mechanism of action.
ACKNOWLEDGEMENT
The authors would like to thank University Grants Commission, New Delhi, for financial assistance (41-125/2012(SR)).
REFERENCES