Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (6): 354 – 358Effects of General and Local Anaesthesia on Innate and Cell–Mediated Immunity in Dogs
Galina Simeonova1, Dinko Dinev1, Maria Andonova2
- Department of Veterinary Surgery, Faculty of Veterinary Medicine, Trakia University, Stara Zagora – 6000, Bulgaria
- Department of General and Clinical Pathology Faculty of Veterinary Medicine, Trakia University, Stara Zagora – 6000, Bulgaria
*Corresponding author: galinavet@abv.bg
ARTICLE CITATION:
Simeonova G, Dinev D, Andonova M (2014). Effects of general and local anaesthesia on innate and cell–mediated immunity in dogs. Adv. Anim. Vet. Sci. 2 (6): 354 – 358.
Received: 2014–06–19, Revised: 2014–07–08, Accepted: 2014–07–08
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.6.354.358
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
The dynamics of innate and specific immunity was investigated in dogs under halothane general anaesthesia and lumbosacral epidural anaesthesia using lidocaine. Phagocytic number (PN), phagocytic index (PI), and nitro blue tetrazolium (NBT) test were used to determine phagocytic properties of neutrophils during both anaesthesia regimens. Specific immunity was assessed by changes in lymphocyte number and distribution of their main subsets. The principal stress hormones were also measured in the blood and their relation with the factors of immune system was evaluated. The results showed an elevation in PN, PI and a reduction in NBT value at 120 min of anaesthesia in both groups, which returned gradually to base values. The changes were, however more pronounced in epidural group. Total lymphocyte number decreased at the cost of B–lymphocytes at 120 min and 24 hours in both groups. On the contrary, CD5+ and CD8+ cells increased in both anaesthesia types at 120 min. General and local anaesthesia produced similar increase in adrenaline, cortisol, and blood glucose. In conclusion, 2 hours of halothane and epidural anaesthesia may stimulate innate immunity and lead to increased number of cells with cytotoxic properties. The immunological disturbances may be attributable to acute stress response induced by the anaesthesia.
INTRODUCTION
Immune system plays a critical role in the progression and outcome of the diseases, but it does not remain intact during anaesthesia and surgery (Hunter, 1999). Suppression of immune function could contribute to postoperative infections (Schneemilch et al., 2004) or to spread of metastases in cancer patients (Thomson, 1987; Snyder and Greenberg, 2010). On the other hand, hyperactive immune response may cause hypersensitivity reactions during or after anaesthesia (Nitti & Nitti, 2002; Armitage–Chan E, 2010). Therefore, the maintenance of immune stability during anaesthesia is of paramount importance for decreasing the perioperative morbidity and mortality.
Neutrophils are the most abundant proinflammatory cell types and have a great impact on the body’s self–defense abilities against bacterial and mycotic infections. Lymphocytes are responsible for the cell–mediated immunity and disturbances in their count and function could lead to autoimmunity diseases or lack of defenses against viruses and foreign particles.
It is well established that surgery and anesthesia may modulate immune response (Kawasaki et al., 2007) but the effect of anaesthesia on patient’s immune system is not fully elucidated.
The aim of the present study was to investigate the alterations in some principal parameters of innate and cell–mediated immunity provoked by local and general anaesthesia in dogs. The relationship between immunological changes and stress response during two types of anaesthesia was also studied.
MATERIALS AND METHODS
Experiment was carried out with the permission of the faculty ethical committee. The study was performed on 21 mongrel dogs of either sex weighing between 14.7 and 20.1 kg and 3 to 5 years of age. The dogs were kept under similar housing and management conditions for acclimatization for a month. The animals were fed with a commercial diet according to the manufacture’s prescriptions and given free access to water. The dogs were dewormed using Prazimec 1 tablet per 10 kg body weight, containing abamectine 2 mg with praziquantel 50 mg (Prazimec DÒ, Biovet, Peshtera, Bulgaria) and vaccinated against canine infectious hepatitis, distemper, leptospirosis, and rabies.
Dogs were divided randomly in three groups. First group was comprised by 8 animals which were submitted to general halothane anaesthesia. Second group consisted of 7 dogs submitted to epidural lumbosacral anaesthesia. Third group included 6 animals and served as a control one. The control (group III) one was used only for blood taking in the same periods of time as the other groups without any treatment in order to eliminate the influence of blood taking and manipulation on the investigated parameters.
The animals in both treatment groups were premedicated with atropine sulfate (Sopharma–Bulgaria) subcutaneously 0.02 mg/kg and acepromazine maleate (CombistressÒ, Kela–Belgium) intramuscularly 0.1 mg/kg, 10 min later.
In the animals of group I general anaesthesia was induced with thiopentone sodium (Biochemie GmbH–Austria) 10 mg/kg administered intravenously as a 2.5% solution 20 min. after acepromazine. Endotracheal intubation was performed and anaesthesia was maintained using halothane (NarcotanÒ, Leciva–Czech Republic) 2.5–3.0% in 100% oxygen (v/v) with flow rate of 2–3L/min. Fluotec Mark III halothane vaporizer mounted on small animal anaesthetic machine and semi closed respiratory circuit were used for the purpose. Halothane was stopped at 120th min after the beginning of premedication but oxygen was given for further 5 min and after the extubation the animals were allowed to recover in quiet room.
In the animals of treatment group II, after subcutaneous infiltration of 2mL 2% lidocaine solution (LidocainÒ, Sopharma–Bulgaria) over the lumbosacral area, epidural anaesthesia was accomplished with the help of a 22–gauge, 6.35 cm Tuohy needle placed in the space between L7 and S1 vertebrae. A catheter was placed through the needle in the epidural space and easy movement of the tip 3–4 cm forward was used as a confirmation of right positioning. Lidocaine 0.3mL/kg was then administered slowly in the epidural space. This dose was sufficient to cause a sensory and motor nerve block up to the level of T5 vertebral segment for a duration of 90 min.
An 18G, 25mm long venous catheter (Vygon GmbH & Co., Germany) was placed in the cephalic vein of the animals in all the groups and saline solution was given at a rate of 10–20mL/kg/h to maintain arterial blood pressure. Jugular vein was also cannulated with an 18G, 45mm long catheter (Vygon GmbH & Co., Germany) in order to withdraw blood samples at specified intervals of anaesthesia.
Blood samples (5 mL) were taken from jugular vein and put in tubes containing Potassium fluride and Sodium ЕDТА 15mM in 1:10 ratio for hormonal and cell count determinations. Venous blood (5 mL) was also taken in tubes containing heparin 50 UI/mL blood for phagocytosis determination. The blood samples were taken just before anaesthesia (0 min) and 30 min, 120 min, 140 min and 24 hours after the beginning of premedication. Cell number was counted at 0 min, 120 min and 24 hour time periods.
- Determination of cell–mediated immunity by:
- Total white blood cell count (WBC) and lymphocyte count (Ly) – using automated cell count analyzer Coulter Electronics, Krefeld, Germany, in G/L.
- Percentage of B–lymphocytes from the total lymphocyte count were defined by zymosan–C3 complement complexes that have connected to the receptor of C3 complement’s component on the surface of B–cells, known as ZC – rosette test (Kajdacvy–Balla & Mendes, 1976), in %.
- Т–lymphocyte subsets CD5+ and CD8+ – after isolation of lymphocytes using separation gradient Histopaque with a density of 1.083 (Sigma Aldrich, St. Luis, MO, USA), their subsets were identified by FITC–conjugated rat monoclonal anti–canine antibodies CD5–clone YKIX 322.3 and CD8–clone YCATE 55.9 (Bio Source International Inc., USA) and visualized by membrane immunofluorescence method, in %.
- Determination of phagocytosis as an indicator of innate immunity. Fluorescein isothyocyanate (FITC) – conjugated Staphylococcus aureus (3.107/mL) and autologous serum were used to realize the process of phagocytosis. Serum application allowed assessing the influence of factors containing in the serum, with opsonic activity toward phagocytes. The ability of cells for phagocytosis was evaluated using the following parameters:
- Phagocyte index (PI) – denoted the percentage of the phagocytes from the total number of neutrophils as determined by immunofluorescence method (Samnaliev et al., 1995) in %.
- Phagocyte number (PN) – denoted the mean number of phagocytized microorganisms by single polymorphonuclear cell as determined by immunofluorescence method (Samnaliev et al., 1995).
- Nitro blue tetrazolium (NBT) reduction test –characterized oxidative respiratory burst of neutrophils, based on the conversion of the soluble colourless nitro blue tetrazolium (NBT) in the insoluble dark blue formasan caused by hydrogen peroxide created during phagocytosis using the method of Park et al. (1968), in %.
- Determination of stress response:
- Adrenalin (ng/mL) was measured by radioimmunological assay using RIA kits (Amersham Biosciences, UK).
- Cortosol (nmol/L) was measured by radioimmunological assay using RIA kits (Amersham Biosciences, UK).
- Glucose (mmol/L) was measured by colorimetrical assay using commercial kit of Human Diagnostica, Germany.
Two–way analysis of variance ANOVA/LSD was used by means of computer program Statmost for Windows, DataMost Corp. 1994–1995 in order to calculate significant differences between time periods as well as between groups. P–value was set at 5%.
RESULTS
White blood cells decreased gradually during halothane anaesthesia starting at 30th min but returned to the baseline at 24 hour (Table 1). A slight and non–significant decrease was found in epidural group. The differences between groups at corresponding intervals were also not significant. Total lymphocyte count decreased insignificantly and temporarily during general and local anaesthesia (Table 2). These low levels were at the expense of B–lymphocytes which decreased markedly at 120 min and 24 hours in both groups, more obviously during general anaesthesia.
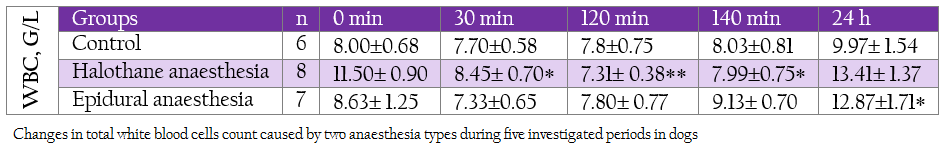
Table 1: Changes in total white blood cells count caused by two anaesthesia types during five investigated periods in dogs
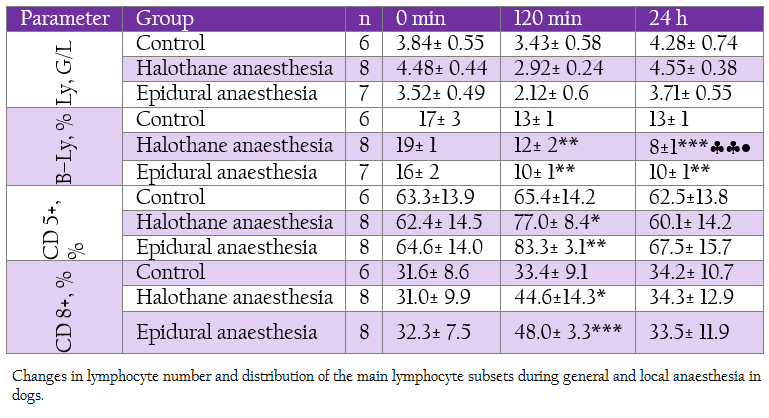
Table 2: Changes in lymphocyte number and distribution of the main lymphocyte subsets during general and local anaesthesia in dogs.
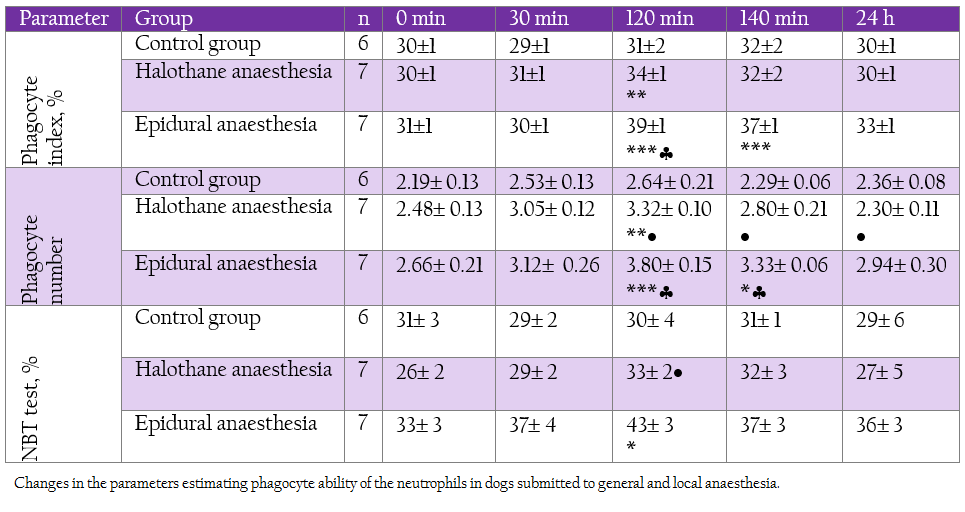
Table 3: Changes in the parameters estimating phagocyte ability of the neutrophils in dogs submitted to general and local anaesthesia.
On the contrary, T–lymphocyte subsets CD 5+ and CD 8+ were elevated during deep halothane and epidural anaesthesia.With regard to neutrophil function all the investigated parameters showed elevation at 120 min and 140 min, more pronounced being in epidural group (Table 3).
Plasma adrenaline levels rose during all investigated periods in both groups. Cortisol concentrations were significantly enhanced at 140 min in both groups. These hormonal changes corresponded with elevation in blood sugar (Table 4).
DISCUSSION
The immunomodulating effects of different anaesthetic agents are multidirectional. Anaesthetics have been suspected of impairing various functions of the immune system either directly, by disturbing the numbers and functions of immune–competent cells, or indirectly by modulating the stress response (Schneemilch et al., 2004; Scholl et al., 2012). Results from the performed investigations in this area showed a lot of contradictions. Some authors reported leukocytosis (Cocelli et al., 2012),
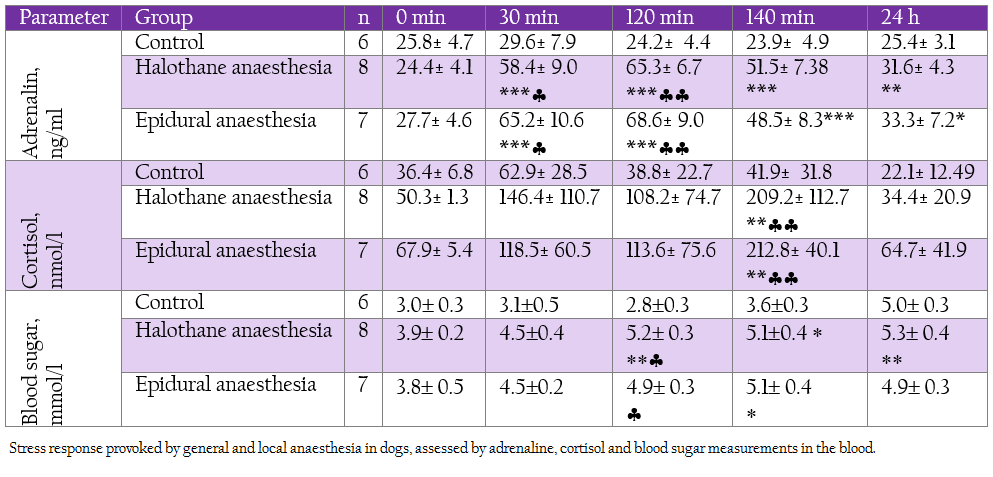
Table 4: Stress response provoked by general and local anaesthesia in dogs, assessed by adrenaline, cortisol and blood sugar measurements in the blood.
while others demonstrated leukopenia (Costa et al., 2013) connected with anaesthesia. Most of the studies on the effects of anaesthesia on the immune function were performed in vitro, or in patients submitted to some kind of surgery. Many in vivo studies have suggested that the immunomodulatory effects of anaesthesia are negligible in comparison to the processes triggered by tissue injuries and pain (Desborough, 2000). Nevertheless, in patients with pre–existing immune dysfunctions, with sepsis and multiple organ failure, as well as in high risk patients, anaesthesia may have undesired consequences (Schneemilch et al., 2004). In the present study, we tried to separate the influence of anaesthesia on the immune system from the influence of surgery in clinical settings.
The first line of defense mechanism of the body is the process of phagocytosis. We found out that epidural anaesthesia and less considerably halothane anaesthesia have an activating effect on the process of phagocytosis by increasing the number of phagocytizing neutrophils, the number of engulfed by them particles, and enhancing respiratory burst. Similar effects were reported in many other studies, showing increased neutrophil count after two hours of inhalation anaesthesia using desflurane and sevoflurane (Cocelli et al., 2012). Halothane anaesthesia also had beneficial effects on the inflammatory response mediated by phagocytes in mice (Colucci et al., 2011). On the contrary, Khan et al. (1995) reported that anaesthesia with halothane caused a dose–related but reversible depression of leucocyte function, assessed by phagocytic index and NBT reduction test in human.
In our study we found out transitional drop in white blood cell count during halothane anaesthesia. The observed leucopoenia was at the expense of lymphocytes as recorded by Costa et al. (2013). This alteration was in unison with elevation in cortisol values starting right at the 30th min but most pronounced at 140th min. This hormone may have a key role in the immunosuppression as it suppress lymphopoiesis and hasten lymphocyte destruction by apoptosis.
The distribution of CD5+ and CD8+ T–cell differed in comparison to the B–lymphocyte subset. In both halothane and epidural anaesthesia these cells increased at 120 min. Contrary to our results are those reported by Cocelli et al. (2012) in human subjected to sevoflurane and desflurane anaesthesia and surgery. They showed an elevation in B–lymphocytes in both groups, increased percentage of CD8 only in desflurane anaesthesia and no change in CD3 positive cells. According to Wei et al. (2013) CD3, CD4 and CD8 T–lymphocyte populations decreased during general and epidural anaesthesia.
CD5 is expressed by most T cells and a small number of B cell. They represent the first line of defense against antigens, have a low activation threshold, and are the only line of defense for those who cannot produce specific antibody. These cells are key regulator of immune response including antitumor response (Fenutria et al., 2011). Abnormalities may produce autoimmunity. CD5 production is elevated in some autoimmune diseases such as rheumatoid arthritis. To our knowledge there were not published surveys about changes in distribution of this cell subset during anaesthesia. CD8 cells have cytotoxic and suppressive T–helpers activities. They can recognise proper antigens and play role in the development of immune tolerance, antitumour and antiviral defence (Snyder and Greenberg, 2010). They are capable to destroy cells that cannot be phagocytised. Opposite to reported response in human, epidural anaesthesia in dogs did not attenuate an elevation in cortisol levels. This might be due to the anxiety of dog caused by disability to move and control caudal part of the body. Hormonal changes that we found during general and local anaesthesia in dogs reflect the activation of stress response of the body towards interference in his internal stability, well expressed in both anaesthesia groups. This effect of anaesthesia is well known and reported in different studies. Taylor, using acepromazine, thiopentone and halothane in sheep (1998) and in ponies (1991) found an increase plasma cortisol and glucose levels and suggested that halothane anaesthesia evokes a stress response which may be associated with cardiovascular depression. But later (Taylor, 1999) after performing plasma expansion during the same anaesthetic protocol, she opined that maintenance of normotension did not entirely depress the response. One of the most significant influences of stress response is on the immune function. We found out that stress response and immunological changes occurred simultaneously during general and local anaesthesia in dogs. Our in vivo results were in contrast to those of Kiefer et al. (2003) who reported that granulocyte phagocytosis activity and generation of reactive oxygen species were reduced by lidocaine in vitro. Acorrding to Ploppa et al. (2008) local anaesthetics have time–dependant suppressive effects on phagocytosis and these effects only occur at concentrations that are unlikely to be routinely attained in clinical settings. Therefore, changes in the immune function could be explained at least partially by the effects of anaesthesiological stress, however other factors, such as pharmacological properties of anaesthetics, may also be implicated in immune disturbances during anaesthesia.
Released stress hormones influence innate as well as cell–mediated immunity. Neutrophil functions increased 10–fold during acute stress replay (Seely et al., 2003) whereas chronic even physiological stress disturb neutrophil microbicidal activity (Tsukamoto and Machida, 2012). Adrenaline stimulates both humoral and cell–mediated immunity by increasing the number of circulating neutrophils and their oxidative killing abilities. The actual mechanism of this effect is demargination of leucocytes and moving of functionally active but, not drowsy cells in the circulation. The trigger process for this effect is binding of released catecholamines to beta–2–adrenergic receptors on the surface of leucocytes and modifying the adhesion molecules (Dimitrov et al., 2010).
Stress hormones have different impact on lymphocytes. The difference in the distribution of lymphocyte subtypes could be explained by the fact that these populations which increased more pronounced during anaesthetic stress such as CD8+ cells, have more dense net of beta–2 receptors as compared to other types. These data suggest that acute stress response during anaesthesia mediated mainly by catecholamines renders beneficial effect on the immune system by increasing cell number responsible for phagocytosis and also increased the proportion of T–cells with suppressive properties. On the other hand, cortisol directly suppresses immune function and if its influence predominates, the stress response turns to have unfavorable effect on the immune system by decreasing the number of defensive cells.
CONCLUSION
Halothane general and lidocaine epidural anaesthesia suppress humoral immunity, therefore, the avoidance of vaccination in the perioperative period is of critical importance. Acute anaesthesia–related stress response could cause favourable influence on the phagocytosis and the cell–mediated immunity by increasing the proportion of CD5 and CD8 cells. The two investigated anaesthesia protocols are not appropriate for immune compromised animals, such as those suffering from autoimmune disorders.
REFERENCES
Armitage–Chan E (2010). Anaphylaxis and anaesthesia. Vet. Anesth. Analg. 37(4): 306–310.
http://dx.doi.org/10.1111/j.1467-2995.2010.00551.x
PMid:20636562
Cocelli LP, Ugur MG, Karadasli H (2012). Comparison of Effects of Low–Flow Sevoflurane and Desflurane Anesthesia on Neutrophil and T–Cell Populations. Curr. Ther. Res. 73(1–2): 42 – 51.
Colucci D, Harvey G, Gayol MC, Elena G, Puig N (2011). Halothane anesthesia in mice: effect on the phagocytic activity and respiratory burst of peritoneal macrophages. Neuroimmunomodulat. 18(1):11 – 18.
http://dx.doi.org/10.1159/000313367
PMid:20606489
Costa PF, Nunes N, Belmonte EA, Moro JV, Lopes PCF (2013). Hematologic changes in propofol–anesthetized dogs with or without tramadol administration. Arq. Bras. Med. Vet. Zoo. 65(5): 1306 – 1312.
http://dx.doi.org/10.1590/S0102-09352013000500007
Desborough JP (2000). The stress response to trauma and surgery. Br. J. Anaesth. 85: 109 – 117.
http://dx.doi.org/10.1093/bja/85.1.109
PMid:10927999
Dimitrov S, Lange T, Born J (2010). Selective Mobilization of Cytotoxic Leukocytes by Epinephrine. J. Immunol. 184 (1): 503 – 511.
http://dx.doi.org/10.4049/jimmunol.0902189
PMid:19949113
Fenutria R, Martinez VG, Gil V, Sintes J, Simôes I, Merino J, Merino R, Ramos–Casals M, Raman C, Engel P, Lozano F (2011). Role of CD5/CD5L interactions in the homeostasis of regulatory lymphocyte subpopulations and the control of autoimmune disorders. J. Transl. Med. 9 (Suppl 2): O6. doi:10.1186/1479–5876–9–S2–O6.
http://dx.doi.org/10.1186/1479-5876-9-S2-O6
Hunter JD (1999). Effects of anaesthesia on the human immune system. Hosp. Med. 60(9): 658 – 663.
http://dx.doi.org/10.12968/hosp.1999.60.9.1198
PMid:10621792
Kajdacsy–Balla AA, Mendes NF (1976). Rosette for mation between human b lymphocytes and zymosan–C3 complexes using different complement sources. J. Immunol. Methods. 9(3–4): 205 – 209.
http://dx.doi.org/10.1016/0022-1759(76)90195-2
Kawasaki T, Ogata M, Kawasaki C, Okamoto K, Sata T (2007). Effects of epidural anaesthesia on surgical stress–induced immunosuppression during upper abdominal surgery. Br. J. Anaesth. 98(2): 196 – 203.
http://dx.doi.org/10.1093/bja/ael334
PMid:17218378
Khan FA, Kamal RS, Mithani CH, Khurshid M (1995). Effect of anaesthesia and surgery on neutrophil function. Anaesthesia. 50(9): 769 – 775.
http://dx.doi.org/10.1111/j.1365-2044.1995.tb06137.x
PMid:7573865
Kiefer RT, Ploppa A, Krueger WA, Plank M, Nohe B, Haeberle HA, Unertl K, Dieterich, HJ (2003). Local anesthetics impair human granulocyte phagocytosis activity, oxidative burst, and CD11b expression in response to Staphylococcus aureus. Anesthesiology. 98(4): 842 – 848.
http://dx.doi.org/10.1097/00000542-200304000-00009
PMid:12657844
Nitti JT, Nitti GJ (2002). Anesthetic complications. In: Morgan GE, Mikhail MS, Murray MJ (eds.) Clinical anesthesiology, 3rd ed., McGraw–Hill Companies Inc., USA, pp.889 – 894.
Park BH, Fikrig SM, Smithwick EM (1968). Infection and nitroblue–tetrazolium reduction by neutrophils. A Diagnostic Acid. Lancet. 2 (7567): 532 – 534.
http://dx.doi.org/10.1016/S0140-6736(68)92406-9
Ploppa A, Kiefer RT, Krueger WA, Unertl KE, Durieux ME (2008). Local anesthetics time–dependantly inhibit staphylococcus aureus phagocytosis, oxidative burst, and CD11b expression by human neutrophils. Reg. Anesth. Pain. Med. 33(4): 297 – 303.
http://dx.doi.org/10.1016/j.rapm.2007.05.012
http://dx.doi.org/10.1097/00115550-200807000-00003
PMid:18675739
Samnaliev M, Mladenov K, Draskova T, Samnalieva T, Padevsky P, Radivanov A (1995). Development and clinical assessment of some nonspecific factors of immunity. In: Proceedings of the First National Congress of Immunology, Sofia, Bulgaria, 1st–3rd November, pp. 135 – 137.
Schneemilch CE, Schilling T, Bank U (2004). Effects of general anaesthesia on inflammation. Best Pract. Res. Cl. An. 18 (3): 493 – 507.
http://dx.doi.org/10.1016/j.bpa.2004.01.002
Scholl R, Bekker A, Babu R (2012). Neuroendocrine and Immune Responses to Surgery. Internet J. Anesth. 30 (3).
Seely AJE, Pascual JL, Christou NV (2003). Cell membrane expression (connectivity) regulates neutrophil delivery, function, and clearance. Crit. Care. 7: 291 – 307.
http://dx.doi.org/10.1186/cc1853
PMid:12930553 PMCid:PMC270693
Snyder GL and Greenberg S (2010). Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br. J. Anaesth. 105(2): 106 – 115.
http://dx.doi.org/10.1093/bja/aeq164
PMid:20627881
Taylor PM (1991). Stress responses in ponies during halothane or isoflurane anaesthesia after induction with thiopentone or xylazine/ketamine. Vet. Anaesth. Analg. 18: 8–14.
http://dx.doi.org/10.1111/j.1467-2995.1991.tb00005.x
Taylor PM (1998). Endocrine and metabolic responses to halothane and pentobarbitone anaesthesia in sheep. Vet. Anesth. Analg. 25: 24–30.
http://dx.doi.org/10.1111/j.1467-2995.1998.tb00165.x
Taylor PM (1999). Effects of plasma expansion on the pituitary–adrenocortical response to halothane anaesthesia in sheep. Vet. Anesth. Analg. 26: 32 – 37.
http://dx.doi.org/10.1111/j.1467-2995.1999.tb00183.x
Thomson DA (1987). Anesthesia and immune system. J. Burn Care Rehabil. 8(6): 483 – 487.
http://dx.doi.org/10.1111/j.1365-2044.1987.tb03994.x
Tsukamoto K, Machida K (2012). Effects of life events and stress on neutrophil functions in elderly men. Immunity and Ageing. 9: 13.
http://dx.doi.org/10.1186/1742-4933-9-13
PMid:22682371 PMCid:PMC3411432
Wei L, Meng QG, Bi ZG (2013). Result of a randomized clinical trial comparing different types of anaesthesia on the immune function of patients with osteosarcoma undergoing radical resection. Panminerva Med. 55(2): 211 – 216.
PMid:23676961




