Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (6): 351 – 353Cytogentic Screening Related to Infertility Problems in Nili–Ravi Buffalo in Punjab
Muhammad Dawood1*, Khalid Javed1, Mubin Mustafa kiyani2, Ahmad Ali3, Muhammad Rifaqat Ameer4, Amjad Iqbal1, Asad Ali1, Ali Haider Saleem1
- Department of Livestock Production, University of Veterinary and Animal Sciences, Lahore
- Faculty of Health & Medical Sciences, Riphah International University Islamabad
- Departmet of Bioscinece COMSATS Institute of Information Technology
- Department of Bioinformatics & Biotechnology, International Islamic University Islamabad
*Corresponding author: muhammad_dawood1989@hotmail.com
ARTICLE CITATION:
Dawood M, Javed K, kiyani MM, Ali A, Ameer MR, Iqbal A, Ali A, Saleem AH (2014). Cytogentic screening related to infertility problems in nili–ravi buffalo in Punjab. Adv. Anim. Vet. Sci. 2 (6): 351 – 353.
Received: 2014–07–10, Revised: 2014–07–21, Accepted: 2014–07–22
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.6.351.353
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
The present study was carried out with the aim of karyotyping and identifying individual chromosome of Nili–Ravi buffalo to document the incidence of chromosomal abnormalities (if any) leading to infertility problems in buffalo. For this purpose blood sample were drawn from 5 normal reproductive buffalo and 30 reported repeat breeder and anestrus Nili–Ravi buffalo. Chromosomal preparations were done through standard whole blood cell culture and harvesting procedure with some modifications according to our lab conditions. After harvesting slides were prepared and stained with Giemsa. Present study revealed that Nili – Ravi buffalo have not numerical chromosomal abnormalities but have some structural abnormalities which may not lead to infertility. In this study we haven’t found any sex chromosome abnormality which is major cause of infertility. It can be concluded that Nili–Ravi buffalo become non-cyclic or repeat breeder due to some other environmental or nutritional problems. Cytogenetic screening can help in early detection of chromosomal abnormalities which may cause infertility and avoid financial losses.
INTRODUCTION
Buffalo has two types: Asiatic and African buffalo (Iannuzzi et al., 2009). The origin of Asiatic Buffalo is subcontinent and this has also two sub classes: river buffalo and swamp buffalo. Total buffalo population in world is more than 168 million; about 161 million of these are in Asia (FAO, 2000). Total River buffalo comprise 11.1% of world bovid population but most of the people’s livelihood depends on water buffalo than any other species (Kierstein et al., 2004). Buffalo milk has more fat, protein and minerals than cow and it comprises 5 percent of the world’s milk supply (FAO, 2000). We have selected Nili–Ravi, Asiatic river buffalo (Bubalus Bubalis) buffalo for the present study. River buffalo have 25 pairs of chromosome. First five pairs are meta/sub metacentric and the remaining are acrocentric (Ali et al., 2001). In 1964 Ingemar Gustavsson was the first who worked on clinical cytogenetic in animals and found first time 1/29 robertsonian translocation in cattle. Some abnormalities include robertsonian translocation in captive Thai Gaur (Chaveerach et al., 2007), in Veitnamese cattle (Tanaka et al., 2000), pericentric inversion of chromosomes and chimeric karyotypes in cattle and buffalo, sex chromosomes reciprocal translocation including XX translocation in Mehsana buffalo (Patel et al., 2006) and bovine freemartin syndrome. Aneuploidy, polyploidy, fragile sites and deletions were observed in cattle (El–Bayomi et al., 2011) Livestock populations have been extraordinarily improved in the last 40 years through cytogenetic screening (Ducos et al., 2008). There are less numbers of cytogenetics and genome studies found in Pakistani buffalo breeds (Ali et al., 2012). No reports on cytogenetic abnormalities in livestock species have so far been observed in Pakistan. With this background information on the status of farm animal cytogenetic in Pakistan this was planned. Main objectives of the study includes the standardization of the lymphocyte culturing and harvesting protocol for Animal Genetic Lab, University of Veterinary & Animal Sciences, Pattoki, Pakistan and also to screen out the infertile buffalo and their new born calves on chromosomal bases to eliminate any carriers of heritable chromosome abnormality which can lead to infertility.
MATERIALS AND METHODS
Geographical Location
This study was conducted in Animal Genetics Lab, Department of Livestock Production, University of Veterinary and Animal Sciences (UVAS), Ravi Campus, Pattoki, Pakistan.
Experimental Animals
Present research was carried out on thirty five number of Nili–Ravi buffalo divided into two groups; group one having 5 phenotypically normal cyclic Nili–Ravi buffalo and group two having reported 30 non–cyclic or repeat breeders Nili–Ravi buffalo. All samples were collected from Buffalo Research Institute Bhoneki, Pattoki, Pakistan. All the buffalos were free from any external or internal parasites or tuberculosis.
Cytogenetic Preparations
For whole blood cell culture the protocol of Ali et al., (2010) followed with some modifications. 3 ml blood from jugular vein of the animal was collected under hygienic conditions. Cytogenetic slides were prepared according to the technique of Henegariu et al., (2001) and stained with Giemsa. Forty good quality metaphases from slides were captured and then karyotyping was done with the help of Olympus BX–51/Genus FISH Imaging system.
RESULTS AND DISCUSSION
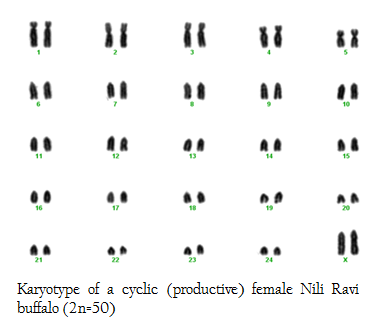
In this research we used 5 phenotypically normal reproductive buffalos to compare with the phenotypically known repeat breeders and anestrus buffalos and also to check the difference between karyotypes of the reproductively abnormal and normal females of river buffalo. Cytogenetic analysis of reproductively normal buffalo revealed that Nili Ravi have diploid 2n=50 (XX), number of chromosomes (Figure 1). Same chromosome numbers and karyotypes were reported by different researches (Ahmed et al., 2004; Ali et al., 2010; Ali et al., 2012). The chromosomal investigation showed that repeat breeder and anestrus buffalo have some structural chromosomal abnormalities but they may not lead to infertility (Figure 2, 3). Our major concerns were related to numerical abnormalities which were not found in selected animals. Results of reported buffalo (repeat breeder and anestrus) revealed that all the studied animals possess normal chromosomes 2n=50 (XX). Fragile site is a chromosomal abnormality having two types; common and rare. Rare fragile sites are very important and they are 5 percent of the total population. Rare site consist of some nucleotide repeats may be two or three and are often prone to impulsive breakage which may affect genes (Durkin and Glover, 2007). In repeat breeder and anestrus animals we have found some fragile sites. Rare fragile sites on sex chromosome may lead to infertility. Mostly numerical abnormalities may affect the sex of the animal and ultimately lead to infertility (Albarella et al., 2013). We have not found any numerical or sex chromosome abnormality in the present study and we can say that Nili–Ravie have not any genetic abnormality related to infertility but it might be mismanagement and poor or mal nutrition which may lead to infertility.
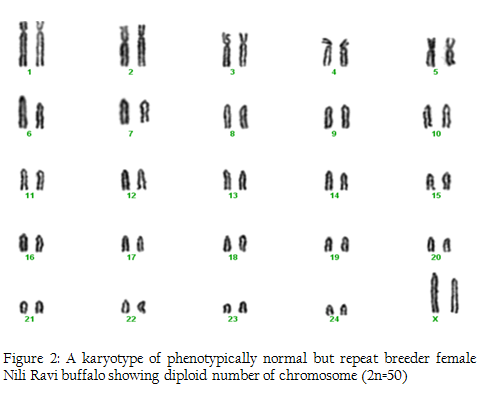
Figure 2: A karyotype of phenotypically normal but repeat breeder female Nili Ravi buffalo showing diploid number of chromosome (2n=50)
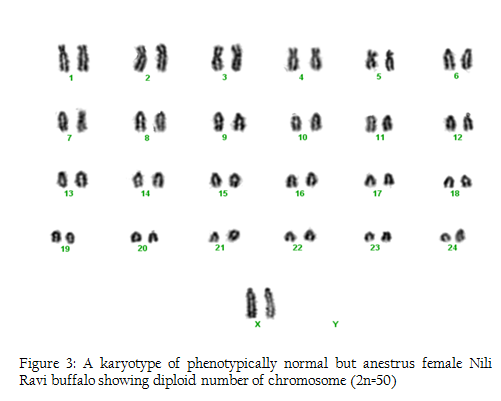
Figure 3: A karyotype of phenotypically normal but anestrus female Nili Ravi buffalo showing diploid number of chromosome (2n=50)
This study was the pilot study regarding chromosomal abnormalities and their identification and relation with infertility problems. This study includes few animals, so that it is proposed that more animal number be used to have more insight of chromosomal abnormalities prevailing in our local livestock population. Cytogenetic has made very impressive development in the livestock sector to identify the abnormalities (structural and numerical) during last 5 decades. Now a day’s cytogenetic techniques have become very important and fundamental part of animal’s sciences in many countries (Nicolae, 2007). After completion of this study a chromosomal screening protocol was established and it might be helpful for screening out our livestock population at early stage and it may reduce the cost of rearing unwanted animals and play vital role in the improvement of livestock production. This technique is cost effective than raising an abnormal individual. That’s why it is suggested that government must have cytogenetic tests at livestock farms and also provide facility to commercial farmers in screening out their abnormal animals.
REFERENCES
Ahmad I, Javed K, Sattar A (2004). Screening of breeding bulls of different breeds through karyotyping. Pak. Vet. J. 24(4): 190 – 192.
Albarella S, Ciotola F, Coletta A, Genualdo V, Iannuzzi L, Peretti V (2013). A New Translocation t (1p; 18) in an Italian Mediterranean River Buffalo (Bubalus bubalis, 2n = 50) Bull: Cytogenetic, Fertility and Inheritance Studies. Cyto. Gen. Res. 139(1): 17 – 21.
http://dx.doi.org/10.1159/000342360
PMid:22986410
Ali A, Abdullah M, Javed K, Babar ME, Mustafa H, Ahmad N, Akhtar M (2012). Cytogenetic and genome studies in Pakistani buffalo (Bubalus bubalis) – A review. J. Anim. Plant Sci. 22(3): 225 – 227.
Ali A, Nawaz M, Babar ME, Aziz M, Akhtar M (2010). First report on standard G–banded karyotype of Nili–Ravi buffalo (Bubalus bubalis). Pak. J. Zool. 42(2): 177 – 180.
Ali S, Ahmad Z, Mohiuddin G, Akhtar P, Ashfaq M (2001). Studies on the karyotype of the Nili–Ravi buffalo. Pak. Vet. J. 21(2): 72 – 76.
Chaveerach A, Wanpen K, Alongkoad T, Wiwat S (2007). New robertsonian translocation chromosomes in captive Thai Gaur (Bos gaurus readei). Pak. J. Biol. Sci. 10(13): 2185 – 2191.
http://dx.doi.org/10.3923/pjbs.2007.2185.2191
PMid:19070179
Ducos A, Revay T, Kovacs A, Hidas A, Pinton A, Bonnet–Garnier A, Molteni L, Slota E, Switonski M, Arruga MV, van Haeringen WA, Nicolae I, Chaves R, Guedes–Pinto H, Andersson M, Iannuzzi L (2008). Cytogentic screening of livestock populations in Europe: an overview. Cyto. Gen. Res. 120(1–2): 26 – 41.
http://dx.doi.org/10.1159/000118738
PMid:18467823
Durkin SG, Glover TW (2007). Chromosome fragile sites. Annu. Rev. Genet. 41: 169 – 192.
http://dx.doi.org/10.1146/annurev.genet.41.042007.165900
PMid:17608616
El–Bayomi KM, Iman EE, Asmaa WZ (2011). Cytogenetic analysis related to some infertility problems in cattle. Global Vet. 7(4): 323 – 329.
FAO (2000). Water Buffalo: an asset undervalued, F.R.O.f.A.a. Bangkok, Thailand: Pacific Editor. 1 – 6.
Gustavsson I, Gunnar R (1964). Chromosome abnormality in three cases of lymphocytic leukaemia in cattle. Nature. 203: 990.
http://dx.doi.org/10.1038/203990a0
PMid:14203527
Henegariu O, Nyla AH, Lisa LW, Patricia BW, David CW, Gail HV (2001). Improvements in cytogenetic slides preparation: Controlled chromosome spreading, chemical aging and gradual denaturing. Cytometery. 43(1): 101 – 109.
Iannuzzi L, Di Meo G (2009). Water Buffalo. Genome Mapping and Genomics in Domestic Animals. 3: 19-31
http://dx.doi.org/10.1007/978-3-540-73835-0_2
Kierstein G, Vallinoto M, Silva A, Schneider MP, Iannuzzi L, Brenig B (2004). Analysis of mitochondrial D–loop region casts new light on domestic water buffalo (Bubalus bubalis) phylogeny. Mol. Phylogenet. Evol. 30(2): 308 – 324.
http://dx.doi.org/10.1016/S1055-7903(03)00221-5
Patel RK, Singh KM, Soni KJ, Chauhan JB (2006). Novel cytogenetic finding: an unusual X; X–translocation in Mehsana buffalo (Bubalus bubalis). Cyto. Gen. Res. 115(2): 186 – 188.
http://dx.doi.org/10.1159/000095241
PMid:17065802
Tanaka K, Yoshi Y, Takashi A, Takahiro Y, Vu–Binh D, Yoichi M, Takao N (2000). A robertsonian translocation, Rob (2; 28), found in Vietnamese cattle. Heriditas. 133(1): 19 – 23.
http://dx.doi.org/10.1111/j.1601-5223.2000.t01-1-00019.x