Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (6): 344 – 350Loop Mediated Isothermal Amplification (LAMP) Test– a Novel Nucleic Acid Based Assay for Disease Diagnosis
Valsala Rekha1*, Rajneesh Rana1, Thachappully Remesh Arun1, Plantharayil Bharathan Aswathi2, Jeny Kalluvila John1, Devi Gopinath1, Gurupriya Vijayasaraswathy Sadanandan1, Aron Jacob1
- Indian Veterinary Research Institute, Izatnagar, Bareilly, UP
- Central Avian Research Institute, Izatnagar, Bareilly, UP
*Corresponding author: rekha14v@gmail.com
ARTICLE CITATION:
Rekha V, Rana R, Arun TR, Aswathi PB, John JK, Gopinath D, Sadanandan GV, Jacob A (2014). Loop mediated isothermal amplification (LAMP) test– a novel nucleic acid based assay for disease diagnosis. Adv. Anim. Vet. Sci. 2 (6): 344 – 350.
Received: 2014–05–26, Revised: 2014–07–14, Accepted: 2014–07–15
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.6.344.350
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Disease diagnosis is of crucial importance in making appropriate therapeutic decisions. Conventionally, diagnosis was based on isolation and identification of the causative agent. This was followed in timeline by the development of serological tests. Nowadays, molecular tests credited with high sensitivity like polymerase chain reaction and its variants are replacing the older diagnostic techniques. Major obstacle in adoption of PCR based technique is the need for thermocycler, which precludes its use in low resource setting areas. Isothermal amplification system like Loop mediated isothermal amplification (LAMP) test is a good alternative. It is a novel nucleic acid amplification assay developed in 2000. Amplification of nucleic acid is carried out under isothermal condition, usually at temperature ranging from 60–65℃. Comparatively higher sensitivity, visual detection, lower reaction time, and dispensable use of thermocycler make LAMP an attractive option for field diagnosis. As the test is not susceptible to inhibitory effect of biological substances, it can be very well applied for direct detection from clinical samples without processing. Moreover, quantitaion of nucleic acid is possible by real time turbidity measurement. Till date, LAMP tests have been developed for diagnosis of a large number of diseases including bacterial, fungal, viral, and parasitic diseases. Instrument–free systems also have come up. In near future, microchip based LAMP system will be commercially available for point of care testing.
INTRODUCTION
Diagnosis of disease is important for adoption of proper therapeutic and prophylactic measures. There has been great revolution in the field of disease diagnosis over time. Earlier the basis for diagnosis was isolation and identification of the etiological agent from clinical specimens. Later, serological tests came up as diagnostic tools, which reduced the time for diagnosis. With the evolution of molecular techniques, the time needed for arriving at final diagnosis was cut down to hours from days.
Diagnostic tests based on nucleic acid amplification serve as valuable diagnostic tools, especially in case of diseases where early diagnosis is important factor in determining the final outcome of disease. Moreover, for many of the diseases, isolation of associated etiological agent may be difficult due to fastidious nature which may pose a major hurdle in arriving at proper diagnosis of the underlying cause. In such situations, nucleic acid based detection methods are promising alternative. In 1983, Karry. B. Mullis conceptualised Polymerase Chain Reaction (PCR) for the cyclic amplification of nucleic acids, while working as chemist for Cetus Corporation. This was an invention that transformed the field of molecular diagnosis to a great extend and he was awarded with Nobel Prize in Chemistry in 1993. In PCR reactions, two specific oligonucleotide primers are used to amplify the target sequence. Repetitive cycles of denaturation, annealing and extension are involved in this, resulting in doubling in the number of target sequences at the end of each cycle. There is an exponential increase in the amount of target sequence over time, as it proceeds through different cycles of amplifications (Mullis et al., 1986). Due to advantages such as increased sensitivity, non–culture based amplification, and reduced time for diagnosis, PCR assays were developed for diagnosis of a number of diseases (Wright and Wynford–Thomas, 1990; Lisby, 1993; Rodriguez, 1997; Yang and Rothman, 2004). Also it can be applied for detection of oncogene, antibiotic resistance, epidemiological studies, and genomic fingerprinting (Versalovic and Lupski, 2002; Jannes and DeVos, 2006; Mothershed and Whitney, 2006, Weile and Knabbe, 2009).
Many variants of PCR have been developed for the diagnostic purposes: Multiplex PCR that facilitates simultaneous detection of multiple sequences (Chamberlain et al., 1988), nested PCR with increased sensitivity using two sets of primers, inverse PCR for the amplification of unknown sequences that flank known sequence (Ochman et al., 1988), real time PCR for quantification of nucleic acid (Higuchi et al., 1993). Despite the advantages of PCR based assays like higher sensitivity, the need for costly thermocycler and post amplification processing preclude its use as routine diagnostic tool in low resource setting laboratories. Rather PCR facilities for accurate diagnosis get restricted to high level specialised diagnostic laboratories.
Several isothermal amplification techniques came up over time: Transcription Mediated Amplification (Guatelli et al., 1990), Strand Displacement amplification (Walker et al., 1992), Rolling Circle Amplification (Fire and Xu, 1995), Helicase Dependent Amplification (Vincent et al., 2004) etc. One of the most widely used isothermal nucleic acid amplification method is Loop mediated isothermal amplification (LAMP). Since its beginning in 2000, LAMP tests have been developed for the detection of a vast variety of disease causing agents. This test has been brought in as an effective alternative diagnostic tool for a number of human, animal as well as plant pathogens. Besides disease diagnosis, this newly developed nucleic acid amplification test has a variety of other applications (Fu et al., 2011). LAMP test can be used for identification of genetically modified organisms – Cauliflower Mosaic Virus 35S promoter gene specific LAMP primers for determining GMO content of Roundup Ready soyabean (Fukuta et al., 2004), GMO specific primers (Lee et al., 2009), GM maize MON863 (Huang et al., 2014); embryonic sex determination (Hirayama et al., 2006; Zoheir and Allam, 2011); meat species identification (Ahmed et al., 2010; Abdulmawjood et al., 2014).
LOOP MEDIATED ISOTHERMAL AMPLIFICATION (LAMP)
Higher sensitivity credited with nucleic acid amplification methods make them attractive options as diagnostic tests. But the need for costly equipment like thermocycler restricts their use at field level and laboratories with less facility (Nirju, 2012). Nowadays diagnostic technologies are transforming from benchside to bedside point of care testing (POCT), facilitating easy and early diagnosis (Niemz et al., 2011). Isothermal amplification methods are good options as bedside diagnostic tests. In 2000, Notomi et al. devised the novel isothermal amplification technique LAMP. The basic principle of this technique is autocycling strand displacement DNA synthesis using specific polymerase enzyme with high strand displacement activity like Bst polymerase. The major advantage associated with LAMP technique is that nucleic acid amplification can be carried out under isothermal conditions in less time as compared to PCR without compromising the sensitivity and specificity. This makes it adoptable for field level diagnosis. Besides, result can be interpreted based on visual detection which is not possible with PCR.
In LAMP reaction, minimum four sets of primers are used: two outer primers (F3, B3) and two inner primers (FIP, BIP). Designing of primers is of prime importance for the efficient amplification. Target sequence of upto 300 bp is taken and four sets of primers that specifically recognize six distinct regions in the target are designed. Different softwares are available for LAMP primer designing– Primer Explorer (Eiken Co.), LAMP Designer (PREMIER Biosoft International) etc. Incorporation of two more primers i.e. loop primers increase the specificity as well as reduce the reaction time (Nagamine et al., 2002). These loop primers bind to stem–loop structures formed during reaction and further accelerate the reaction.
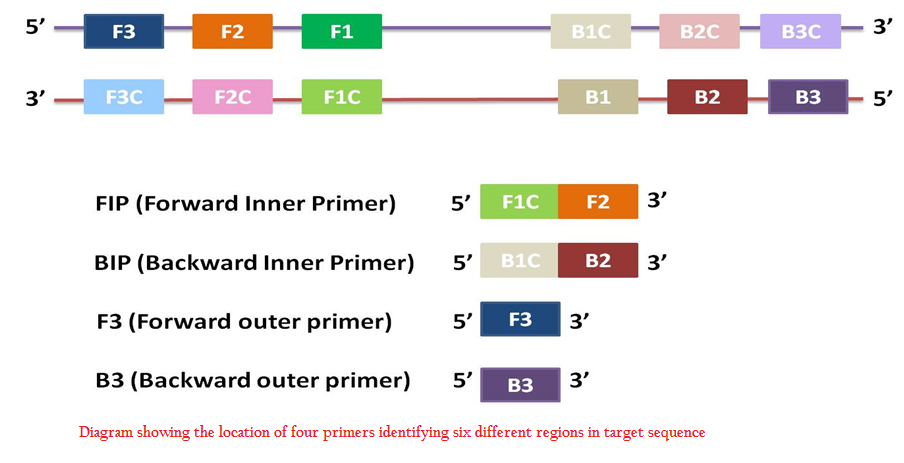
Figure 1: Diagram showing the location of four primers identifying six different regions in target sequence
Major challenge in taking up molecular detection for field level diagnosis is sample preparation for extraction of nucleic acid as PCR is susceptible to inhibitors present in biological samples. The tolerance of LAMP to PCR inhibitors make it suited for direct detection from samples (Kaneko et al., 2007). During the last decade, there have been many reports of development of LAMP tests for disease diagnosis. In the early years after the inception of concept of LAMP, the focus was on the validation of test for identification of different pathogens. But now the interest has shifted to miniaturization of the test in order to make it suited for field level or point of care testing (Mori et al., 2013).
LAMP can be used for amplification of DNA as well as RNA (Laohasinnarong, 2011). Quantitation is possible similar to real time PCR by real time analysis of turbidity using turbidimeter (Mori et al., 2004). It is cheaper as compared to real time PCR in which fluorescent probes are used. But the turbidity is independent of amplified sequence unlike fluorescent probe. For simultaneous detection of multiple targets, multiplexing can be done (Tanner et al., 2012; Mahony et al., 2013; Yamazaki et al., 2013).
Several LAMP based diagnostic kits are available for disease diagnosis– Legionella, Giardia, verotoxin producing Escherichia coli, E. coli O157:H7, Campylobacter, Salmonella, Listeria monocytogenes etc (http://loopamp.eiken.co.jp/e/products/index.html). These kits are commercialised by Eiken chemical company, Tokyo. The need for preservation of reagents at lower temperatures is another obstacle in low resource setting areas. Reagents made available in freeze dried form can overcome this (http://www.freepatentsonline.com/y2008/0182312.html). Such kits are available for diagnosis of tuberculosis, malaria and African trypanosomiasis, produced by FIND (Foundation for Innovative New Diagnostics) in collaboration with Eiken Company (http://www.finddiagnostics.org/programs/).
PRINCIPLE OF LAMP
LAMP reaction mixture consists of DNA polymerase with high strand displacement activity i.e. Bst polymerase, four sets of primers– two inner primers and two outer primers, deoxy nucleotide triphosphates dNTPs, magnesium sulphate, betaine, buffer for enzyme, and template DNA. Usually reaction is carried out at 60–65℃ for one hour. The target sequence consists of six distinct regions namely F3, F2, F1, B1c, B2c, and B3c in order from 5’ to 3’ end. Two outer primers are F3 and B3. Inner primers contain the sequence of both sense and antisense strands. FIP contains F1C and F2. Similarly BIP contains B1C and B2 (Figure 1).
Inner primers are added in higher concentration as compared to outer primers. Reaction gets initiated by binding of FIP to complementary sequence in target DNA, which is followed by strand displacement synthesis by outer primer F3. This produces FIP linked complementary strand, with loop at one end. BIP will bind on it, followed by B3. Thus results in the production of a dumb bell shaped structure with loops at both ends. By self primed synthesis, it produces stem loop structure, which acts as starting material for cyclic amplification step. Subsequently FIP binds to it and initiates synthesis by strand displacement producing stem–loop DNA with inverted copy of target sequence at stem and loop involving BIP at other end. On this structure, BIP binds and produces stem loop structure with twice long stem. This process continues. Final product of reaction is a mixture of stem loop DNAs of varying lengths and cauliflower like structures with multiple loops (Notomi et al., 2000). Loop primers will bind to loop regions that are not bound by inner primers (Nagamine et al., 2002).
DETECTION OF LAMP PRODUCT
The foremost advantage of LAMP in comparison with PCR is that the result of amplification can be interpreted without post amplification processing. Visual turbidity is a good indicator of positive reaction. Nucleic acids are amplified in large amount in LAMP reaction. This results in production of large excess of pyrophosphate ions, which will combine with magnesium ions resulting in production of white precipitate of magnesium pyrophosphate. This is responsible for turbidity in case of positive reaction (Mori et al., 2001). Increase in turbidity will be in direct proportion to the amount of nucleic acid, which can be measured real time using real time turbidimeter. Turbidity is measured as OD at 400nm in every 6 seconds. It is cheaper than real time PCR machine. For the formation of white precipitate, yield in microgram quantities is required. In LAMP, DNA is amplified to more than 10µg, therefore visual turbidity can be used as an indicator of positive reaction (Parida et al., 2008).
Polyethylene imine (PEI) can be added to reaction tube post amplification for the detection of amplification. PEI forms insoluble complex with high molecular weight amplification product, but will not combine with low molecular weight oligonucleotides. Visually detectable clear coloured precipitate is formed on addition of PEI. But PEI cannot be added prior to reaction, as it will inhibit amplification (Mori et al., 2006).
Amplified products can also be visualised in presence of fluorescent intercalating dyes such as SYBR Green I, Calcein etc. On addition of SYBR Green I to the reaction tube post amplification, the colour changes from orange to green in case of positive reaction. Fluorescence can be detected visually using handheld UV torch (wavelength 365nm). Calcein is a fluorescent metal ion indicator. It can be added to tubes prior to reaction. Calcein quenches the manganous ions. Before reaction, solution appears orange in colour. As the reaction proceeds, manganous ions are released from calcein and it will combine with pyrophosphate ions, thus increasing fluorescence of calcein. Increased fluorescence can be detected visually as well as by ultraviolet light (Tomita et al., 2008).
Colorimetric detection is also possible by addition of 120μM Hydroxynaphthol blue (HNB) to the reaction mix before amplification. HNB is a metal ion indicator. The colour of HNB changes depending on pH of the solution. Positive reaction is indicated by change in colour from violet to sky blue (Goto et al., 2009).
Analysis of LAMP reaction products can be done by agarose gel electrophoresis on 2% agarose gel, followed by staining with ethidium bromide solution and visualisation under UV transilluminator. In positive cases, it will produce ladder like pattern due to the production of stem–loop structures with different stem lengths (Parida et al., 2008). Either restriction enzyme digestion or sequencing can be done for the confirmation of specificity of the amplified product in LAMP reaction.
APPLICATIONS OF LAMP AS A DIAGNOSTIC TEST
The shorter reaction time without compromising the sensitivity and specificity, and the independence from the use of thermocycler makes the test suitable for diagnosis in low facility laboratory settings, which cannot afford the high cost equipments. LAMP tests have been designed for the diagnosis of a vast array of diseases (Table 1).
INSTRUMENT FREE LAMP SYSTEMS AS POCT
According to WHO, point of care diagnostic test must be ASSURED (Affordable, Sensitive, Specific, Rapid and robust, Equipment free, and Deliverable to the end user). Isothermal amplification techniques can be adopted for field diagnosis in equipment free systems, since there is no need for thermocycling. Instrument free, microLAMP systems are
well suited for diagnosis in low resource setting areas. Non–Instrumented Nucleic acid Amplification (NINA) for malaria was developed by LaBarre et al. in 2011. They devised NINA heater for carrying out LAMP reaction on the basis of heat generation from exothermic reaction between calcium oxide and water. In order to maintain the temperature, reaction chamber was surrounded with engineered fat substance with melting range at 65℃. Temperature can be maintained for 45 minutes with reaction between 20g calcium oxide and 6.8ml water, which is enough for one LAMP reaction. Result can be interpreted based on turbidity or fluorescent dyes. The major hurdle in adoption of nucleic acid amplification assays as field level diagnostics is the requirement of nucleic acid extraction. LAMP is well suited for direct detection from clinical samples (Kaneko et al., 2007). There have been reports of direct detection of Plasmodium falciparum from blood samples by just heating at 99℃ for 10 minutes prior to LAMP reaction (Poon et al., 2006). For diagnosis of tuberculosis in developing countries, LAMP for direct detection from sputum samples has been developed by FIND (Foundation for Innovative New Diagnostics) in collaboration with Eiken Co (Boehme et al., 2007). In this, sputum samples are subjected to pretreatment for 7–8 minutes to remove the inhibitory substances and then they are directly added to freeze dried LAMP reagents (PURE–TB LAMP).
ADVANTAGES AND DISADVANTAGES
Following are the points in credit for loop mediated isothermal amplification test as diagnostic test over PCR. Dispensable use of thermocycler makes the test less expensive. Result is obtained in less than 1 hour. Sensitivity is higher than that of conventional PCR. Non–denatured template can be used for amplification (Nagamine et al., 2001). Amplification is produced under isothermal conditions and can be performed either in less equipped laboratories or at field level, in a water bath. Amplicon can be detected visually using hydroxy naphthol blue, SYBR Green I etc. LAMP is credited with tolerance to inhibitory substances such as culture medium and biological substances which can affect the efficiency of PCR (Kaneko et al., 2007).
Inspite of these advantages, there are some drawbacks associated with this technique. Most important problem is the chance of carry over contamination, since nucleic acid is amplified to microgram quantities. By avoiding post amplification opening of the tube, contamination chances can be lessened to some extent. For this, dyes like HNB, calcein etc. can be added to the reaction mixture prior to amplification, as these dyes will not interfere with amplification process. Another strategy is UTP/UNG system used for avoiding PCR carry over contamination. Preincubation with enzyme UNG (Uracil N– Glycosylase) which will degrade the uracil and use of UTP instead of TTP in deoxyribonucleotide mix in reaction prevents contamination from previously amplified products (Dhama et al., 2014). Sample preparation, amplification reactions, and post amplification processing (if needed) must be carried out in different rooms to avoid chances of contamination at all stages. Another disadvantage is that LAMP cannot be used for amplification of sequences of size more than 300bp.
CONCLUSION
In present time, more focus is given to molecular level detection for disease diagnosis. A variety of molecular diagnostic tests have come up in the last few decades. Loop mediated isothermal amplification (LAMP) assay is one of the most important innovative tests developed in the last decade. Since it is carried out under isothermal conditions and result can be interpreted visually, it is well suited for adoption as a field level diagnostic in low resource setting areas in developing countries. Reaction components can be made available in freeze dried form, which will preclude the need for low temperature storage. Various modifications of LAMP like equipment free systems (NINA) have been made for making it suited for point of care testing. In coming time, microchip based LAMP test kits combining nucleic acid extraction, LAMP amplification and detection will be coming up for major animal and human diseases. This will pave a new opening for field level diagnosis in less time with high sensitivity and specificity.
REFERENCES
Abdulmawjood A, Grabowski N, Fohler S, Kittler S, Nagengast H, Klein G (2014). Development of Loop–Mediated Isothermal Amplification (LAMP) Assay for Rapid and Sensitive Identification of Ostrich Meat. PLoS ONE 9: e100717. doi:10.1371/journal.pone.0100717
http://dx.doi.org/10.1371/journal.pone.0100717
Ahmed MU, Hasan Q, Hossain MM, Saito M, Tamiya E (2010). Meat species identification based on the loop mediated isothermal amplification and electrochemical DNA sensor. Food Control. 21: 599 – 605.
http://dx.doi.org/10.1016/j.foodcont.2009.09.001
Alhassan A, Thekisoe OMM, Yokoyama N, Inoue N, Motloang MY, Mbati PA, Yin H, Katayama Y, Anzai T, Sugimoto C, Igarashi I (2007). Development of loop–mediated isothermal amplification (LAMP) method for diagnosis of equine piroplasmosis. Vet. Parasitol. 143: 155 – 160.
http://dx.doi.org/10.1016/j.vetpar.2006.08.014
PMid:16973284
Angamuthu R, Baskaran S, Gopal DR, Devarajan J, Kathaperumal K (2012). Rapid detection of the Marek's disease viral genome in chicken feathers by loop–mediated isothermal amplification. J. Clin. Microbiol. 50: 961 – 965.
http://dx.doi.org/10.1128/JCM.05408-11
PMid:22170920 PMCid:PMC3295103
Bai Z, Shi L, Hu C, Chen X, Qi J, Ba X, Peng Q, Chen Y, Chen H, Guo A (2011). Development of a loop–mediated isothermal amplification assay for sensitive and rapid detection of Mycoplasma bovis. Afr. J. Biotechnol. 10: 12333 – 12338.
Boehme CC, Nabeta P, Henostroza G, Raqib R, Rahim Z, Gerhardt M, Sanga E, Hoelscher M, Notomi T, Hase T, Perkins MD (2007). Operational feasibility of using loop–mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J. Clin. Microbiol. 45: 1936 – 1940.
http://dx.doi.org/10.1128/JCM.02352-06
PMid:17392443 PMCid:PMC1933042
Chamberlain JS, Gibbs RA, Ranier JE, Nguyen PN, Caskey CT (1988). Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 16: 11141 – 11156.
http://dx.doi.org/10.1093/nar/16.23.11141
PMid:3205741 PMCid:PMC339001
Chen MX, Ai L, Zhang RL, Xia JJ, Wang K, Chen SH, Zhang YN, Xu MJ, Li X, Zhu XQ, Chen JX (2011). Sensitive and rapid detection of Paragonimus westermani infection in humans and animals by loop–mediated isothermal amplification (LAMP). Parasitol. Res. 108: 1193 – 1198.
http://dx.doi.org/10.1007/s00436-010-2162-x
PMid:21107864
Das A, Babiuk S, McIntosh MT (2012). Development of a Loop–Mediated Isothermal Amplification Assay for Rapid Detection of Capripoxviruses. J. Clin. Microbiol. 50: 1613 – 1620.
http://dx.doi.org/10.1128/JCM.06796-11
PMid:22357504 PMCid:PMC3347125
Dhama K, Karthik K, Chakraborthy S, Tiwari R, Kapoor S, Kumar A, Thomas P (2014). Loop–mediated isothermal amplification of DNA (LAMP): a new diagnostic tool lights the world of diagnosis of animal and human pathogens: a review. Pak. J. Biol. Sci. 17: 151 – 166.
http://dx.doi.org/10.3923/pjbs.2014.151.166
PMid:24783797
Endo S, Komori T, Ricci G, Sano A, Yokoyama K, Ohori A, Kamei K, Franco M, Miyaji M, Nishimura K (2004). Detection ofgp43 of Paracoccidioides brasiliensis by the loop–mediated isothermal amplification (LAMP) method. FEMS Microbiol. Lett. 234: 93 – 97.
http://dx.doi.org/10.1111/j.1574-6968.2004.tb09518.x
PMid:15109725
Fire A, Xu SQ (1995). Rolling replication of short DNA circles. Proc. Natl. Acad. Sci. USA. 92: 4641 – 4645.
http://dx.doi.org/10.1073/pnas.92.10.4641
PMid:7753856 PMCid:PMC42000
Fu S, Qu G, Guo S, Ma L, Zhang N, Zhang S, Gao S, Shen Z (2011). Applications of loop–mediated isothermal DNA amplification. Appl. Biochem. Biotechnol. 163: 845 – 850.
http://dx.doi.org/10.1007/s12010-010-9088-8
PMid:20844984
Fukuda S, Takao S, Kuwayama M, Schimazu Y, Miyazaki K (2006). Rapid detection of Norovirus from fecal specimens by Real–time Reverse Transcription Loop–mediated isothermal amplification assay. J. Clin. Microbiol. 44: 1376 – 1381.
http://dx.doi.org/10.1128/JCM.44.4.1376-1381.2006
PMid:16597865 PMCid:PMC1448634
Fukuta S, Mizukami Y, Ishida A, Uaeda J, Hasegawa M, Hayashi I, Hashimoto M, Kanbe M (2004). Real–time loop–mediated isothermal amplification for the CaMV–35S promoter as a screening method for genetically modified organisms. Eur. Food Res. Technol. 218: 496 – 500.
http://dx.doi.org/10.1007/s00217-003-0862-5
Gao M, Cui J, Ren Y, Suo S, Li G, Sun X, Su D, Opriessnig T, Ren X (2012). Development and evaluation of novel reverse transcription loop–mediated isothermal amplification (RT–LAMP) assay for detection of type II porcine reproductive and respiratory syndrome virus. J. Virol. Methods. 185: 18 – 23.
http://dx.doi.org/10.1016/j.jviromet.2012.05.016
PMid:22659065
Goto M, Honda E, Ogura A, Nomoto A, Hanaki K (2009). Colorimetric detection of loop–mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 46: 167 – 172.
http://dx.doi.org/10.2144/000113072
PMid:19317660
Guatelli JC, Whitfield KM, Kwoh DY, Barringer KJ, Richman DD, Gingeras TR (1990). Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc. Natl. Acad. Sci. USA. 87: 1874 – 1878.
http://dx.doi.org/10.1073/pnas.87.5.1874
http://dx.doi.org/10.1073/pnas.87.19.7797-a
PMid:2308948 PMCid:PMC53586
Han ET, Watanabe R, Sattabongkot J, Khuntirat B, Sirichaisinthop J, Iriko H, Jin L, Takeo S, Tsuboi T (2007). Detection of four Plasmodium species by genus– and species–specific loop–mediated isothermal amplification for clinical diagnosis. J. Clin. Microbiol. 45: 2521 – 2528.
http://dx.doi.org/10.1128/JCM.02117-06
PMid:17567794 PMCid:PMC1951264
Higuchi R, Fockler C, Dollinger G, Watson R (1993). Kinetic PCR analysis: real–time monitoring of DNA amplification reactions. Biotechnology (N Y). 11: 1026 – 1030.
http://dx.doi.org/10.1038/nbt0993-1026
Hill J, Beriwal S, Chandra I, Paul VK, Kapil A, Singh T, Wadowsky RM, Singh V, Goyal A, Jahnukainen T, Johnson JR, Tarr PI, Vats A (2008). Loop–mediated isothermal amplification assay for rapid detection of common strains of Escherichia coli. J. Clin. Microbiol. 46: 2800 – 2804.
http://dx.doi.org/10.1128/JCM.00152-08
PMid:18550738 PMCid:PMC2519505
Hirayama H, Kageyama S, Takahashi Y, Moriyasu S, Sawai K, Onoe S, Watanabe K, Kojiya S, Notomi T, Minamihashi A (2006). Rapid sexing of water buffalo (Bubalus bubalis) embryos using loop–mediated isothermal amplification. Theriogenology. 66: 1249 – 1256.
http://dx.doi.org/10.1016/j.theriogenology.2006.03.036
PMid:16672158
Huang S, Xu Y, Yan X, Shang Y, Zhu P, Tian W, Xu W (2014). Development and application of a quantitative loop–mediated isothermal amplification method for detecting genetically modified maize MON863. J. Sci. Food Agric. doi: 10.1002/jsfa.6707
http://dx.doi.org/10.1002/jsfa.6707
Ina’cio J, Flores O, Spencer–Martins I (2008). Efficient identification of clinically relevant Candida yeast species by use of an assay combining panfungal loop–mediated isothermal DNA amplification with hybridization to species–specific oligonucleotide probes. J. Clin. Microbiol. 46: 713 – 720.
http://dx.doi.org/10.1128/JCM.00514-07
PMid:18077626 PMCid:PMC2238081
Iwamoto T, Sonobe T, Hayashi K (2003). Loop–mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J. Clin. Microbiol. 41: 2616 – 2622.
http://dx.doi.org/10.1128/JCM.41.6.2616-2622.2003
PMid:12791888 PMCid:PMC156570
Jannes G, DeVos D (2006). A review of current and future molecular diagnostic tests for use in the microbiology laboratory. Methods Mol. Biol. 345: 1 – 21.
PMid:16957343
Ji H, Li H, Zhu L, Zhang H, Wang Y, Zuo Z, Guo W, Xu Z (2012). Development and evaluation of a loop–mediated isothermal amplification (LAMP) assay for rapid detection of Actinobacillus pleuropneumoniae based the dsbE–like gene1. Pesq. Vet. Bras. 32: 757 – 760.
http://dx.doi.org/10.1590/S0100-736X2012000800014
Kaneko H, Kawana T, Fukushima E, Suzutani T (2007). Tolerance of loop–mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods. 70: 499 – 501.
http://dx.doi.org/10.1016/j.jbbm.2006.08.008
PMid:17011631
Karanis P, Thekisoe O, Kiouptsi K, Ongerth J, Igarashi I, Inoue N (2007). Development and preliminary evaluation of a loop–mediated isothermal amplification procedure for sensitive detection of Cryptosporidium oocysts in fecal and water samples. Appl. Environ. Microbiol. 73: 5660 – 5662.
http://dx.doi.org/10.1128/AEM.01152-07
PMid:17616628 PMCid:PMC2042060
LaBarre P, Hawkins KR, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Boyle D, Weigl B (2011). A simple, inexpensive device for nucleic acid amplification without electricity–toward instrument–free molecular diagnostics in low–resource settings. PLoS One. 9: e19738.
http://dx.doi.org/10.1371/journal.pone.0019738
PMid:21573065 PMCid:PMC3090398
Laohasinnarong (2011). Loop–mediated Isothermal Amplification (LAMP): An Alternative Molecular Diagnosis. J. Appl. Anim. Sci. 4: 10 – 19.
Lee D, LaMura M, Allnutt TR, Powell W (2009). Detection of genetically modified organisms (GMOs) using isothermal amplification of target DNA sequences. BMC Biotechnol. 9: 7 – 13.
http://dx.doi.org/10.1186/1472-6750-9-7
PMid:19187544 PMCid:PMC2656497
LeRoux CA, Kubo T, Grobbelaar AA, van Vuren PJ, Weyer J, Nel LH, Swanepoel R, Morita L, Paweska JT (2008). Development and evaluation of Real–time Reverse Transcription Loop–mediated isothermal amplification assay for rapid detection of Rift valley fever virus in clinical samples. J. Clin. Microbiol. 47: 645 – 651.
http://dx.doi.org/10.1128/JCM.01412-08
PMid:19109471 PMCid:PMC2650915
Li C, Chen J, Shi H, Zhang X, Shi D, Han X, Chi Y, Feng L (2014). Rapid detection of porcine kobuvirus in feces by reverse transcription loop–mediated isothermal amplification. Virol. J. 11: 73 – 78.
http://dx.doi.org/10.1186/1743-422X-11-73
PMid:24755372 PMCid:PMC4026823
Li J, Minion FC, Petersen AC, Jiang F, Yang S, Guo P, Li J, Wu W (2013). Loop–mediated isothermal amplification for rapid and convenient detection of Mycoplasma hyopneumoniae. World J. Microb. Biot. 29: 607 – 616.
http://dx.doi.org/10.1007/s11274-012-1216-x
PMid:23184577
Lin GZ, Zheng FY, Zhou JZ, Gong XW, Wang GH, Cao XA, Qiu CQ (2011). Loop–mediated isothermal amplification assay targeting the omp25 gene for rapid detection of Brucella spp. Mol. Cell. Probes. 25: 126 – 129.
http://dx.doi.org/10.1016/j.mcp.2011.01.001
PMid:21232598
Lin JZ, Zheng FY, Zhou JZ, Wang GH, Cao XA, Gong XW, Qiu CQ (2012). Loop–mediated isothermal amplification assay targeting the MOMP gene for rapid detection of Chlamydia psittaci abortus strain. Pak. Vet. J. 32: 273 – 276.
Lisby G (1993). Polymerase chain reaction: use in microbiological diagnosis. Ugeskr Laeger. 155: 1708 – 1712.
PMid:8317014
Mahony J, Chong S, Bulir D, Ruyter A, Mwawasi K, Waltho D (2013). Multiplex loop–mediated isothermal amplification (M–LAMP) assay for the detection of influenza A/H1, A/H3 and influenza B can provide a specimen–to–result diagnosis in 40 min with single genome copy sensitivity. J. Clin. Virol. 58: 127 – 131.
http://dx.doi.org/10.1016/j.jcv.2013.06.006
PMid:23827787
Mori Y, Hirano T, Notomi T (2006). Sequence specific visual detection of LAMP reactions by addition of cationic polymers. BMC Biotechnol. doi: 10.1186/1472–6750–6–3
http://dx.doi.org/10.1186/1472-6750-6-3
Mori Y, Kanda H, Notomi T (2013). Loop–mediated isothermal amplification (LAMP): recent progress in research and development. J. Infect. Chemother. 19: 404 – 411.
http://dx.doi.org/10.1007/s10156-013-0590-0
PMid:23539453
Mori Y, Kitao M, Tomita N, Notomi T (2004). Real–time turbidimetry of LAMP reaction for quantifying template DNA. J. Biochem. Biophys. Methods. 59: 145 – 157.
http://dx.doi.org/10.1016/j.jbbm.2003.12.005
PMid:15163526
Mori Y, Nagamine K, Tomita N, Notomi T (2001). Detection of loop– mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 289: 150 – 154.
http://dx.doi.org/10.1006/bbrc.2001.5921
PMid:11708792
Mothershed EM, Whitney AM (2006). Nucleic acid–based methods for the detection of bacterial pathogens: Present and future considerations for the clinical laboratory. Clin. Chim. Acta. 363: 206 – 220.
http://dx.doi.org/10.1016/j.cccn.2005.05.050
PMid:16139259
Mullis K, Faloona E, Scharf S, Saiki R, Horn G, Erlich H (1986). Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp. Quant. Biol. 51: 263 – 273.
http://dx.doi.org/10.1101/SQB.1986.051.01.032
PMid:3472723
Nagamine K, Watanabe K, Ohtsuka K, Hase T, Notomi T (2001). Loop–mediated isothermal amplification reaction using a nondenatured template. Clin. Chem. 47: 1742 – 1743.
PMid:11514425
Nagamine K, Hase T, Notomi T (2002). Accelerated reaction by loop–mediated isothermal amplification using loop primers. Mol. Cell. Probes. 16: 223 – 229.
http://dx.doi.org/10.1006/mcpr.2002.0415
PMid:12144774
Niemz A, Ferguson TM, Boyle DS (2011). Point–of–care nucleic acid testing for infectious diseases. Trends Biotechnol. 29: 240 – 250.
http://dx.doi.org/10.1016/j.tibtech.2011.01.007
PMid:21377748 PMCid:PMC3746968
Nirju ZK (2012). Loop–mediated isothermal amplification technology: towards point of care diagnostics. PLoS Negl. Trop. Dis. 6: e1572. doi:10.1371/journal.pntd.0001572
http://dx.doi.org/10.1371/journal.pntd.0001572
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000). Loop–mediated isothermal amplification of DNA. Nucleic Acids Res. 28: e63.
http://dx.doi.org/10.1093/nar/28.12.e63
PMid:10871386 PMCid:PMC102748
Ochman H, Gerber AS, Hartl DL (1988). Genetic applications of Inverse Polymerase Chain Reaction. Genetics. 120: 621 – 623.
PMid:2852134 PMCid:PMC1203539
Parida M, Posadas G, Inoue S, Hasebe F, Morita K (2004). Real–time Reverse Transcription Loop–mediated isothermal amplification for rapid detection of West Nile virus. J. Clin. Microbiol. 42: 257 – 263.
http://dx.doi.org/10.1128/JCM.42.1.257-263.2004
PMid:14715762 PMCid:PMC321710
Parida M, Sannarangaiah S, Dash PK, Rao PVL, Morita K (2008). Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 18: 407 – 421.
http://dx.doi.org/10.1002/rmv.593
PMid:18716992
Parida M, Santhosh SR, Dash PK, Tripathi NK, Lakshmi V, Mamidi N, Shrivastva A, Gupta N, Saxena P, Babu JP, Rao PVL, Morita K (2007). Rapid and real–time detection of Chikungunya virus by reverse transcription loop–mediated isothermal amplification assay. J. Clin. Microbiol. 45: 351–357.
http://dx.doi.org/10.1128/JCM.01734-06
PMid:17135444 PMCid:PMC1829040
Poon LLM, Wong BWY, Ma EHT, Chan KH, Chow LMC, Abeyewickereme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S, Peiris JSM (2006). Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat–treated blood by loop–mediated isothermal amplification. 52: 303 – 306.
Qiao YM, Guo YC, Zhang XE, Zhou YF, Zhang ZP, Wei HP, Yang RF, Wang DB (2007). Loop–mediated isothermal amplification for rapid detection of Bacillus anthracis spores. Biotechnol. Lett. 29: 1939 – 1946.
http://dx.doi.org/10.1007/s10529-007-9472-9
PMid:17673950
Rodriguez JM (1997). Detection of animal pathogens by using the polymerase chain reaction (PCR). Vet. J. 153: 287 – 305.
http://dx.doi.org/10.1016/S1090-0233(97)80063-9
Saleh M, Soliman H, El–Matbouli M (2008). Loop–mediated isothermal amplification as an emerging technology for detection of Yersinia ruckeri the causative agent of enteric red mouth disease in fish. BMC Vet. Res. 4: 31 – 41.
http://dx.doi.org/10.1186/1746-6148-4-31
PMid:18700011 PMCid:PMC2531098
Scheel CM, Zhou Y, Theodoro RC, Abrams B, Balajee SA, Litvintseva AP (2014). Development of a loop–mediated isothermal amplification method for detection of Histoplasma capsulatum DNA in clinical samples. J. Clin. Microbiol. 52: 483 – 488.
http://dx.doi.org/10.1128/JCM.02739-13
PMid:24478477 PMCid:PMC3911334
Song Q, Wang L, Fang R, Khan MK, Zhou Y, Zhao J (2012). Detection of Mycoplasma wenyonii in cattle and transmission vecctrs by the loop–mediated isothermal amplification (LAMP) assay. Trop. Anim. Health Prod. 45: 247 – 250.
http://dx.doi.org/10.1007/s11250-012-0197-y
PMid:22684637
Su J, Liu X, Cul H, Li Y, Chen D, Li Y, Yu G (2014). Rapid and simple detection of methicillin–resistance Staphylococcus aureus by orfX loop–mediated isothermal amplification assay. BMC Biotechnl. 14: 8.
http://dx.doi.org/10.1186/1472-6750-14-8
PMid:24456841 PMCid:PMC3902190
Sun D, Wang J, Wu R, Wang C, He X, Zheng J, Yang H (2010). Development of a novel LAMP for visible detection of swine Pasteurella multocida. Vet. Res. Commun. 34: 649 – 657.
http://dx.doi.org/10.1007/s11259-010-9433-y
PMid:20717843
Tanner NA, Zhang Y, Evans Jr. TC (2012). Simultaneous multiple target detection in real–time loop–mediated isothermal amplification. Biotechniques. 53: 81 – 89.
PMid:23030060
Thekisoe OMM, Inoue N, Kuboki N, Tuntasuvan D, Bunnoy W, Borisutsuwan S, Igarashi I, Sugimoto C (2005). Evaluation of loop–mediated isothermal amplification (LAMP), PCR and parasitological tests for detection of Trypanosoma evansi in experimentally infected pigs. Vet. Parasitol. 130: 327 – 330.
http://dx.doi.org/10.1016/j.vetpar.2005.04.019
PMid:15908123
Tomita N, Mori Y, Kanda H, Notomi T (2008). Loop–mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 3: 877 – 882.
http://dx.doi.org/10.1038/nprot.2008.57
PMid:18451795
Venkatesan G, Bhanuprakash V, Balamurugan V, Singh RK, Pandey AB (2012). Development of loop–mediated isothermal amplification assay for specific and rapid detection of camelpox virus in clinical samples. J. Virol. Methods. 183: 34 – 39.
http://dx.doi.org/10.1016/j.jviromet.2012.03.019
PMid:22575686
Versalovic J, Lupski JR (2002). Molecular detection and genotyping of pathogens: more accurate and rapid answers. Trends Microbiol. 10: 15 – 21.
http://dx.doi.org/10.1016/S0966-842X(02)02438-1
Vincent M, Xu Y, Kong H (2004). Helicase–dependent isothermal DNA amplification. EMBO Rep. 5: 795 – 800.
http://dx.doi.org/10.1038/sj.embor.7400200
PMid:15247927 PMCid:PMC1249482
Walker GT, Little MC, Nadeau JG, Shank DD (1992). Isothermal in vitro amplification of DNA by a restriction enzyme/DNA polymerase system. Proc. Natl. Acad. Sci. USA. 89: 392 – 396.
http://dx.doi.org/10.1073/pnas.89.1.392
PMid:1309614 PMCid:PMC48243
Wang D, Zhang G, Lu C, Deng R, Zhi A, Guo J, Zhao D, Xu Z (2011). Rapid detection of Listeria monocytogenes in raw milk with loop–mediated isothermal amplification and chemosensor. J. Food. Sci. 76: 611 – 615.
http://dx.doi.org/10.1111/j.1750-3841.2011.02383.x
PMid:22416713
Wang J, Cheng S, Yi L, Cheng Y, Yang S, Xu H, Li Z, Shi X, Wu H, Yan X (2013). Detection of mink enteritis virus by loop–mediated isothermal amplification (LAMP). J. Virol. Methods. 187: 401 – 405.
http://dx.doi.org/10.1016/j.jviromet.2012.11.012
PMid:23183142
Weile J, Knabbe C (2009). Current applications and future trends of molecular diagnostics in clinical bacteriology. Anal. Bioanal. Chem. 394: 731 – 742.
http://dx.doi.org/10.1007/s00216-009-2779-8
PMid:19377839
Wright PA, Wynford–Thomas D (1990). The polymerase chain reaction: miracle or mirage? A critical review of its uses and limitations in diagnosis and research. J Pathol. 162: 99 – 117.
http://dx.doi.org/10.1002/path.1711620203
PMid:2250198
Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, Xia CM (2010). Sensitive and rapid detection of Schistosoma japonicum DNA by loop–mediated isothermal amplification (LAMP). Int. J. Parasitol. 40: 327 – 331.
http://dx.doi.org/10.1016/j.ijpara.2009.08.010
PMid:19735662
Yamazaki W, Mioulet V, Murray L, Madi M, Haga T, Misawa N, Horii Y, King DP (2013). Development and evaluation of multiplex RT–LAMP assay for rapid and sensitive detection of foot–and–mouth disease. J. Virol. Methods. 192: 18 – 24.
http://dx.doi.org/10.1016/j.jviromet.2013.03.018
PMid:23583488
Yamazaki W, Taguchi M, Ishibashi M, Kitazato M, Nukina M, Misawa N, Inoue K (2008). Development and evaluation of a loop–mediated isothermal amplification assay for rapid and simple detection of Campylobacter jejuni and Campylobacter coli. J. Med. Microbiol. 57: 444 – 451.
http://dx.doi.org/10.1099/jmm.0.47688-0
PMid:18349363
Yang JL, Zhang SH, Liu ZH, Yang R, Huang Y, Wen M (2012). Development and evaluation of loop–mediated isothermal amplification assay for the rapid detection of porcine cytomegalovirus under field conditions. Virol. J. 9: 321. doi:10.1186/1743–422X–9–321.
http://dx.doi.org/10.1186/1743-422X-9-321
Yang S, Rothman RE (2004). PCR–based diagnostics for infectious diseases: uses, limitations, and future applications in acute–care settings. Lancet Infect. Dis. 4: 337 – 348.
http://dx.doi.org/10.1016/S1473-3099(04)01044-8
Zhang H, Thekisoe OMM, Aboge GO, Kyan H, Yamagishi J, Inoue N, Nishikawa Y, Zakimi S, Xuan X (2009). Toxplasma gondii: Sensitive and rapid detection of infection by loop–mediated isothermal amplification (LAMP) method. Exp. Parasitol. 122: 47 – 50.
http://dx.doi.org/10.1016/j.exppara.2009.01.012
PMid:19545521
Zoheir KM, Allam AA (2011). A rapid improved method for sexing embryo of water buffalo. Theriogenology. 76: 83 – 87.
http://dx.doi.org/10.1016/j.theriogenology.2011.01.020
PMid:21396688