Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (1S): 32 – 36Special Issue – 1 (2014) (Infectious Diseases of Animals and Global Health)
Therapeutic Management of Bovine Brucellosis in Endemically Infected Dairy Cattle Herd of Native Sahiwal Breed
Shoor Vir Singh1*, Vivek Kumar Gupta1, Ashok Kumar1, Saurabh Gupta1, Ruchi Tiwari2, Kuldeep Dhama3
- Microbiology Laboratory, Animal Health Division, Central Institute for Research on Goats, Makhdoom, PO– Farah, Mathura (UP)
- Department of Veterinary Microbiology and Immunology, Pandit Deen Dayal Upadhayay Pashu Chikitsa Vigyan Vishvidhyalaya Ewam Go–Anusandhan Sansthan (DUVASU), Mathura (U.P.)–281001
- Division of Pathology, Indian Veterinary Research Institute, Izatnagar (IVRI), Bareilly (U.P.) – 243122
*Corresponding author: shoorvir.singh@gmail.com; shoorvir_singh@rediffmail.com
ARTICLE CITATION:
Singh SV, Gupta VK, Kumar A, Gupta S, Tiwari R, Dhama K (2014). Therapeutic management of bovine brucellosis in endemically infected dairy cattle herd of native sahiwal breed. Adv. Anim. Vet. Sci. 2 (1S): 32 – 36.
Received: 2014–04–25, Revised: 2014–04–28, Accepted: 2014–04–28
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.1s.32.36
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
In–view of the ban on cow slaughter and old system of ‘permanent quarantine houses’ for cows infected with Brucellosis and TB/JD is now non–existent, hence potential of ‘therapeutic schedules’ was evaluated for ‘therapeutic management and cure’ of bovine brucellosis in endemically infected dairy cattle herd of important native breed (Sahiwal), known for high milk production. Twenty seven Sahiwal cows of a dairy herd with history of abortions and still–births, were investigated and studied for 10 weeks following treatment. Brucella abortus was isolated from vaginal discharges of aborted cows and was characterized morphologically, biochemically and on molecular tests. Cows naturally infected were treated in two phases, cows in phase I of therapeutic schedule A were given streptomycin, isoniazid and rifampicin with long acting tetracyclines and cows in therapeutic schedule B were given streptomycin and rifampicin with enrofloxacin for 15 days. In phase II, maintenance schedule A was given isoniazid and rifampicin with long acting tetracyclines and in therapeutic schedule B cows were given isoniazid and rifampicin for 15 days with one shot of bayrocin. Sero–conversion of treated cows was monitored for a period of 10 weeks using SAT and in–house ELISA tests. Cows provided therapeutic schedule A and B, showed reduction in titers of anti–Brucella antibodies. The titers of infection were similar in all cows (>1:80) which were treated. Of the 27 cows treated with two therapeutic schedules, 12 became pregnant and 10 (83.3%) had normal calving. Except two cows, which were in advance stage of pregnancy at the time of treatment, there was no abortion in treated cows. In naturally infected cows the above two schedule though costly proved effective for therapeutic management of Brucella infection.
INTRODUCTION
Many developed countries have eradicated Brucella abortus from their cattle herds, however infection is endemic in developing countries like India due to huge population of domestic livestock, poor sanitary conditions, inadequate management practices, porous borders, minimal attention on sero–surveys, monitoring and control of chronic and zoonotic diseases like brucellosis. Lack of government policies resulted increased incidence of abortions and still births in large remnants. Due to absence of data on National prevalence, bovine brucellosis has emerged major zoonotic infection in Indian domestic livestock in general and dairy cattle herds in particular (Singh et al., 1998; Singh et al., 2013; Singh et al., 2014). Bovine Brucellosis has become a serious problem in Indian cattle dairy herds because of certain religious, social and animal husbandry practices (Singh et al., 2014). Despite extensive studies on different aspects of disease in dairy cattle in past 50 years data on ‘optimum antibiotic treatment’ for the therapeutic management of brucellosis is either not available or is still disputed. This may be due to intracellular localization of Brucella and its ability to adapt to the environmental conditions encountered in it’s ‘replicative niche’ e.g. macrophage, treatment failure and relapse rates are high and depend on the drug combinations and high cost of treatment. Brucellosis in cattle is usually caused by biovars of Brucella abortus. In some countries, particularly in Southern Europe and Western Asia, where cattle are kept in close association with sheep or goats, infection can also be caused by B. melitensis (Jimenez de Bagues, 1991; Verger, 1985). Bovine brucellosis, being zoonotic remains a serious obstacle in public health, social and economic progress, food security and food safety in developing countries, where appropriate preventive and control measures are not in place (Singh et al., 2014). Expansion of animal industries and urbanization and lack of hygienic measures in animal husbandry and in food handling partly account for brucellosis remains a public health hazard.
In an earlier study Hashemi et al. (2012) found that doxycycline–streptomycin was superior to doxycycline–rifampicin. In human beings, recent trials assessed the effect of quinolone based combination therapy and triple drug regimens. Streptomycin has been replaced by newer amino–glycosides and their effects on brucellosis have not been further reported (Skalsky et al., 2008). Finally, the advantage of combination therapy over mono–therapy has not been quantified. In the present study, we performed systematic treatment of naturally infected cattle herd with bovine brucellosis and assessed efficacy of two antibiotics schedules for the therapeutic management of brucellosis and to identify optimal therapeutic model and duration of antibiotic schedule. Present study aimed to identify therapeutic regimen which is effective, practical, without side–effects and relatively inexpensive, in order to make therapeutic management of Brucella infected cows with superior genes, a viable alternative to euthenization and burial due to ban on cow slaughter.
MATERIALS AND METHODS
History
The dairy cattle herd under study consisted of 40 animals (27 adult female cows of 2 to 12 years of age, 10 calves of less than 1.2 years old and 3 adult male bulls of 2 to 3 years of age) of Sahiwal cows, an important high milk yielding native breed of cows. Of the 10 calves, 6 were born blind with eyes normal. Young bulls (2 years old), were purchased from the home tract of Sahiwal breed in neighbouring Haryana state. Nine adult cows (8 to 12 years of age) were procured locally from the local animal markets and brokers. Rest 18 young cows of 2 to 7 years of age were born in the dairy herd. Dairy cattle herd belonged to Sri Krishna Janamsthan Trust located at the birth place of Lord Krishna in Mathura city of Uttar Pradesh. Dairy cows were reared under intensive system (stall fed) and were confined in two sheds (50X35 feet each) and two paddocks (2000 square feet each). Cows were given vaccination against Foot and Mouth Disease and Harmorrhagic Septicaemia and cows in heat were bred by natural service. At the time of visit all animals were suffering with ticks infestation. Six cows were suffering with acute mastitis for past one month and was a common affecting milking cows at the dairy farm. Frequently, large quantities of milk were discarded due to mixing of blood and was not fit for human consumption. Seven cows had suffered from episodes of abortions and still births. Six calves were blind since birth and eyes of the calves were normal but unable to see. Dairy herd was never vaccinated for bovine brucellosis.
Clinical Samples
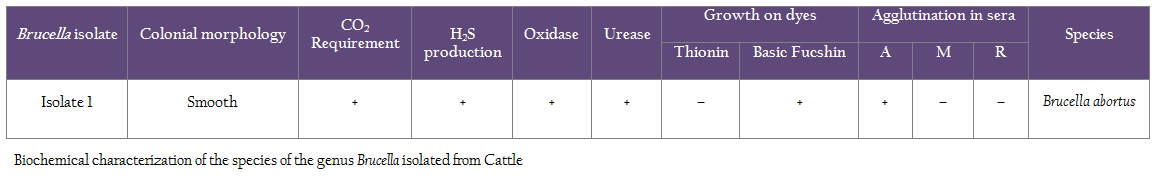
Serum samples were collected from 20 adult cows with history of Brucella induced abortions. Blood samples were collected by jugular veni–puncture. Serum samples were stored at –20oC till further use. To confirm the infection mucus discharge from the vagina of recently aborted cows was collected using sterile swabs. First sampling (serum and mucus discharge) was done on 20.01.2012 of 10 cows with history of abortions. Second sampling (serum and mucus discharge) of all adult 33 animals (27 cows and 3 bulls and 3 young heifers) were sampled. Serum samples were processed by SAT and ELISA and mucus discharge for culture and PCR. Milk samples of 9 cows in milk at the time of visit were also collected in order to attempt isolation of Brucella abortus. Before commencement of the therapeutic schedule, Brucella abortus biovar 1 and 2 were repeatedly isolated from milk and vaginal secretions of recently aborted cows. All 20 cows were positive for Brucella infection by Standard Tube Agglutination test (SAT) and ELISA using Brucella abortus antigen. Serum samples from six Brucella–free cows (negative in bacterial isolation as well as serology) served as negative controls and cows positive in bacterial isolation as well as serology served as positive controls and were used to calculate the cutoff value of the assay.
Enzyme–linked Immunosorbent Assay (ELISA)
Polystyrene plates (96 wells) were coated with Brucella abortus LPS antigen (0.1µg/well) diluted in phosphate–buffered saline (PBS). Unbound sites in the plates were blocked with 200 µL of PBS containing 3% bovine serum albumin (BSA) per well. After the wells were washed with PBS containing 0.05% Tween 20 (PBST), sera were diluted in PBST containing 1% BSA and dispensed to the wells. Specific antibodies were detected with Rabbit anti–bovine HRPO IgG conjugate. The reaction was developed by adding ortho–phenylenediamine (2 µg/µL) in 0.1 M citrate–phosphate buffer containing 0.03% H2O2. To establish the cutoff values of the assays, serum samples from non–infected controls were tested under the same conditions. Cutoff values of each enzyme–linked immune–sorbent assay (ELISA) system were calculated as the mean specific OD of control sera plus 2 standard deviations (SD).
Standard Tube Agglutination Test (SAT)
Cattle were screened by Standard tube agglutination test following the procedure of Alton (1970) and Wright and Smith, (1987) with some modifications using Brucella abortus plain antigen. The agglutination titres of ≥1:40 were considered positive for brucellosis.
Primer Design and PCR
Primer pairs used to identify Brucella spp. at genus specific level include primers for sequences encoding BCSP 31 (B4/B5) (Baily et al 1992), 16S rRNA (F4/R2) (Romero et al., 1995). Amplification reaction mixtures were prepared in volumes of 50 µL containing 1X PCR buffer, 1.5 mM MgCl2, 200 µM each deoxynucleoside triphosphate, 1 µM each primer, 200 ng of genomic DNA and 2.5 U of DNA polymerase. Cycling conditions for the amplification was as follows: initial denaturation was at 94°C for 5 min, followed by 35 cycles at 94°C for 1 min (denaturation), 58°C for 1 min (annealing), 72°C for 2 min (extension), and the final extension at 72°C for 10 min. The amplified product of specific size (223 bp) was considered positive using 0.8% agarose gel.
Therapeutic Schedule A and B
Two therapeutic schedules in two phases were evaluated in 27 cows naturally infected with Brucella abortus. In phase I of treatment schedule A, a combination of oxy–tetracycline (OTC), 30 mL per day by intramuscular route, streptomycin (ST, 6 grams per day for 15 days by intramuscular route) and Rifampicin and Isoniazid (RFI), 6 tabs (600mG + 300mG each) daily by oral route was used. In treatment schedule B, Enrofloxacin (En, 30 mL per day for 15 days by intramuscular route) was used in place of OTC, along with ST and RFI with same dose rate.
In phase II of maintenance schedule A, a combination of oxytetracycline (OTC), 30 mL every third day by intramuscular route and Rifampicin and Isoniazid (RFI), 6 tabs (600mG + 300mG each) daily by oral route was used for 15 days. In phase II of maintenance schedule B, Bayrocin (one shot, 30 mL by intramuscular route) was used in place of Enrofloxacin, along with RFI with same dose rate for 15 days. In both the groups, a herbal liver protective powder (Liv–52 powder) was administered orally with the dose rate of 10 grams daily per cow.
RESULTS AND DISCUSSION
Use of broad–spectrum antibiotics such as aureomycin, terramycin, tetracyclines and streptomycin (ST), singly or in combination, has resulted in the reduction of abortions in infected herds or individual cows (King et al., 1952, Larsen et al., 1950). However, cost of therapy, presence of antibiotic residues in milk and failure to cure udder infections in many cases led to the general conclusion that such treatment may not be suitable for the control of bovine brucellosis, especially in countries where cow slaughter is not prohibited. With the development of Oxytetracycline (OTC) and long–acting (LA)–OTC, the use of these agents alone or in combination with ST has succeeded in eliminating the symptoms of this disease and reducing shedding of brucellae by infected cows at parturition. Consequently, such therapeutic schedules have been applied in infected herds to prevent abortions and reduce spread of brucellosis within herds. However, these schedulesr had limited success in complete cure of infection. Furthermore, serious local reactions were reported in cows, due to repeated intra–peritoneal (i.p.) inoculations with OTC (Fensterbank, 1976; Fensterbank et al., 1975; Milward et al., 1984; Nicolette et al., 1985; Phillippon et al., 1971). In this study also one cow developed lameness due to repeated injections of OTC in the gluteal muscles of hind right limb of cow number 7. In addition, the regimens previously tried involved small doses and few injections of the antibiotics used as compared to the present study. In one of these studies, injections of LA–OTC [20 mG/kg intramuscularly (i.m.) every 3 days for 2 weeks] combined with ST [20 mG/kg (i.m.) daily for 1 week] resulted in–cessation of shedding of Brucella abortus in 10 (71.4%) of 14 cows (Milward et al., 1984). In another study, inoculation of LA–OTC (20 mG/kg i.m. every 3 days for 2 weeks) alone was successful in curing 3 (21.0%) of 14 cows. However, when LA–OTC was combined with ST [20 mG/kg intravenously (i.v.) or intramuscularly (i.m.) daily for 1 week] 14 (67.0%) of 21 cows were successfully treated (Nicolette et al., 1985). Gold standard for diagnosis of Brucellosis remains isolation of Brucella spp. bacteria from vaginal secretion and milk samples. However, PCR–based methods that identify nucleic acid fragments from the bacteria are more useful and practical. Most of the new methods for Brucella spp. identification and typing are still being developed and still await validation for use in clinical samples. We have used PCR for the direct detection of Brucella organisms in clinical specimens from the cows. Target gene used for identification of Brucella abortus at genus level was 16s rRNA which resulted in amplification 1400 bp PCR product because it is conserved in all Brucella species. For species level identification, PCR target sequence of the order of 223 bp present on a gene encoding a 31–kDa Brucella abortus antigen was selected for amplification (Figure 1). Primers B4 and B5 used to amplify the target sequence were described previously by Baily et al. (1992). In both the cases a positive amplification was obtained which indicated presence of Brucella abortus in the animals.
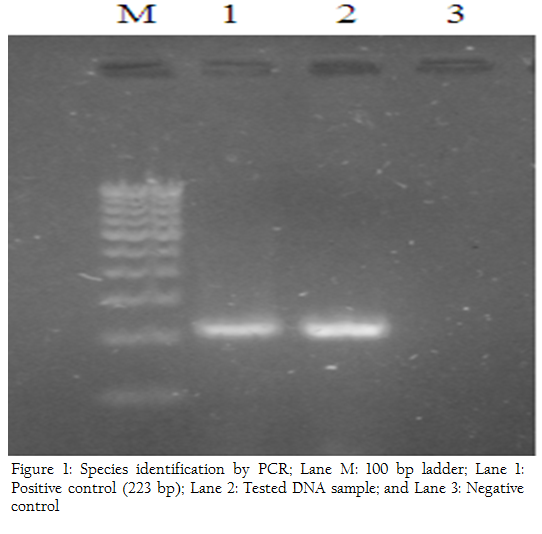
Figure 1: Species identification by PCR; Lane M: 100 bp ladder; Lane 1: Positive control (223 bp); Lane 2: Tested DNA sample; and Lane 3: Negative control
Several chemo–therapeutic agents have been employed in recent decades for the treatment of Brucella abortus infection in cows; however, none of these has been entirely successful. Numerous chemical agents, general antimicrobials (phenols or dyes), trace elements, minerals and mixtures of vitamins (A and E) have been tried unsuccessfully. Furthermore, attempts using antibiotics such as penicillin or sulfonamides failed to cause cessation of the shedding of Brucella abortus from the mammary secretions of infected cows, or caused only temporary cessation (Berman et al., 1946; Dumaresq, 1940).
When Brucella organisms could no longer be recovered from udder secretions or from any of the selected tissue specimens collected from treated animals, the respective cows were considered to be cured (successful treatment). Continued or resumed shedding of Brucella organisms in udder secretions or the isolation of Brucella organisms from any of the tissue specimens obtained were considered as conclusive evidence of treatment failure.
A comparison was made between breeding performance in the herd prior to identification of the infected cows and that in the treated cows in the breeding season following treatment. Before treatment, cows had been living in an infected environment for periods between 1 and 8 years and the rates of abortions and infertility in the herds were very high. In addition, there were unusually high rates of metritis, retained placenta, mastitis with milk production reduced to half and there was also problem of infestation of ticks. However, following treatment, cows were living in an uninfected environment. Of the 27 cows treated with both the regimens, 12 became pregnant and 10 (83.3%) had normal calving. No abortions were observed in the treated cows except two cows, since these cows were in advance stage of pregnancy during treatment schedule. On the whole, treated cows did not show any sign of difficult labor and retained placenta. All the cases of mastitis in the dairy cows got treated due to high antibiotic therapy and milk production of cows showed improvements and was within the normal range for treated cows. Similarly the problem of ticks was also vanished after this treatment regimen from the herd completely and was one of the major health problem of dairy herd before treatment.
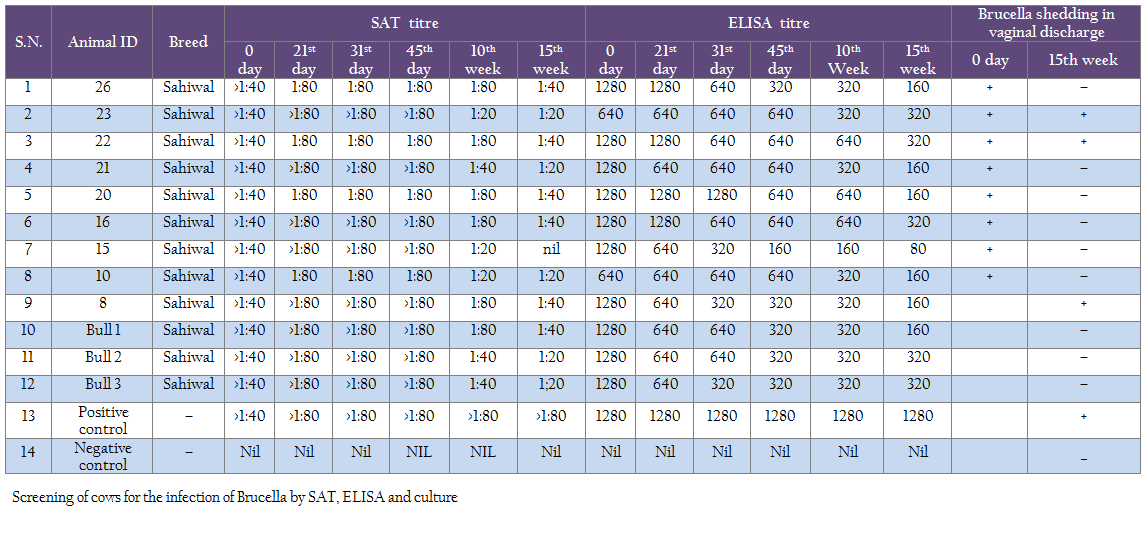
Monitoring of treated cows by standard tube agglutination test showed decrease in titer (1:80 to 1:40 and finally to 1:20 in some cases) from beginning of treatment to 10th and 15th weeks post–treatment (Table 2). Using ELISA test also the titer showed decreasing trend from 31st day to 15th week time period from 1:1280 to 1:160 (Table 2). However none of the cows became sero–negative cows in screening at 15th week of post treatment of cows. While screening of calves born to the mothers after treatment regimen, showed that all calves born from treated cows were sero–negative for brucellosis.
Screening of the vaginal secretions by culture at 10th week after initiation of the antibiotic therapy from all the successfully treated cows in the present study exhibited that shedding of Brucella organisms in vaginal secretions ceased and did not recommence.
‘Test and cull’ method combined with calf–hood vaccination contributes to considerable reduction in the number of infected herds; however, for economic and social reasons (ban on cow slaughter) this method cannot be applied in India in case of cows. An eradication campaign through culling and slaughter and compensation is not within the economic scope of developing countries like India, especially where it is difficult to change the social and religious habits. On the other hand, mass vaccination of infected herds protects only uninfected animals without altering the course of infection. Infected and vaccinated animals continue to present serious public health risk. In addition, average cost of a healthy cow is approximately Rs. 40–50 thousands. If this cow suffers with abortion or still birth it causes serious losses to dairy herd owner. Subsequent problems of irregular breeding, loss of milk production and reduced productivity resulting from spread of infection (Shepered et al., 1979). Moreover, with the development and widespread use of techniques such as artificial insemination and embryo transfer, cattle with superior genetic potential have become increasingly valuable. Slaughter of infected animals with superior genetic potential has serious economic and genetic consequences. Developing countries may not afford the traditional ‘test and slaughter’ approach used and recommended by developed countries. Effective, practical and safe antibiotic therapy would be of enormous benefit to producers in these countries as an alternative to slaughter of infected high value animals.
Doses and duration of application of selected antibiotics in the present study indicate that the long–term therapy and the drug combinations used yielded better results. Both regimens were equally effective in eliminating Brucella organisms from all cows involved in the tests and no relapses were recorded. Total cost of treatment of cows was nearly Rs. 1, 50,000.0 in this period.
CONFLICT OF INTEREST
No conflict of Interest to declare.
ACKNOWLEDGEMENT
Authors are thankful to Director, Central Institute for Research on Goats (CIRG), Makhdoom for providing laboratory facilities.
REFERENCES
Alton, G.G., 1970. Caprine Brucellosis. FAO/WHO Expert Committee on Brucellosis. Document Bruce WP/70.7.
Baily GG, Krahn JB, Drasar BS, Stoker NG (1992). Detection of Brucella melitensis and Brucella abortus by DNA amplification. J. Trop. Med. Hyg. 95: 271–275.
PMid:1495123
Berman DT, Irwin MR, Beach BA (1946). The effect of penicillin on cows infected with Brucella abortus. Cornell Vet. 36: 311–313.
PMid:20289305
Dumaresq JA (1940). Effect of sulfanilamide on Brucella abortus infection in guinea pigs and cows. Aust. Vet. J. 16: 102–107.
http://dx.doi.org/10.1111/j.1751-0813.1940.tb01294.x
Fensterbank R (1976). Traitement de vachesatteintes de brucelloseancienne par l'oxytétracycline. Ann. Rech. Vet. 7: 231–240.
PMid:829211
Fensterbank R, Plommet M, Pardon P (1975). Traitement de la brucellose bovine parlloxytetracycline. Ann. Rech. Vet. 6: 43–66.
Hashemi HS, Gachkar L, Keramat F, Mamani M, Hajilooi M, Janbakhsh A, Majzoobi MM, Mahjub H (2012). Comparison of doxycycline–streptomycin, doxycycline–rifampin and ofloxacin–rifampin in the treatment of brucellosis: a randomized clinical trial. Int. J. Infect. Dis. 16(4): e247–e251.
http://dx.doi.org/10.1016/j.ijid.2011.12.003
PMid:22296864
Jimenez de Bauges MP, Marin CM, Blasco JM (1991). Effect of antibiotic therapy and strain 19 vaccination on the spread of Brucella melitensis within an infected dairy herd. Prev. Vet. Med. 11: 17–24.
http://dx.doi.org/10.1016/S0167-5877(05)80041-8
King NB, Venzke WG, Edgington BH (1952). Studies on the experimental treatment of bovine brucellosis. Am. J. Vet. Res. 13: 152–157.
PMid:14924131
Larsen PH, Gilman HL (1950). Aureomycin as a treatment of acute brucellosis of cattle. Cornell Vet. 40: 259–272.
PMid:15427316
Milward FW, Nicoletti P, Hoffman E (1984). Effectiveness of various therapeutic regimens for bovine brucellosis. Am. J. Vet. Res. 45: 1825–1828.
PMid:6208830
Phillippon A, Plommet M, Renoux G (1971). Brucellose bovine expérimentale: VII. Influence surl'évolution de l'infectiond'unefaible concentration doxytetracycline danslorganisme au moment de l'inoculation. Ann. Rech. Vet. 2: 147–157.
Romero C, Gamazo C, Pardo M, Lopez–Goni I (1995). Specific detection of Brucella DNA by PCR. J. Clin. Microbiol. 33: 615–7.
PMid:7538508 PMCid:PMC227999
Shepherd AA, Simpson BH, Davidson RM (1979). An economic evaluation of the New Zealand bovine brucellosis eradication scheme. International Symposia on Veterinary Epidemiology and Economics proceedings, ISVEE 2: Veterinary Epidemiology and Economics, Proceedings of the 2nd International Symposium, Canberra, Australia, Regional & national economic studies of disease control programs session. 443–447.
Singh SV, Singh N, Gupta VK, Shankar H, Vihan VS, Gupta VK, Tiwari HA (1998). Sero–prevalence of brucellosis in a few important Indian goat breeds. Small Rumin. Res. 30: 93–98.
http://dx.doi.org/10.1016/S0921-4488(98)00094-7
Singh SV, Chaubey KK, Gupta S, Gupta VK, Agrawal ND, Kumar N (2013). Co–infection of Mycobacterium avium subspecies paratuberculosis and Brucella melitensis in a sirohi breed of goats in India. Adv. Anim. Vet. Sci. 1 (6): 188–190.
Singh SV, Yadav RK, Gupta VK, Gupta S, Chaubey KK, Agarwal ND and Kumar N (2014). Co–incidence of bovine Johne's disease and bovine brucellosis in young bulls of murrah breed in their native tract (Rohtak, Haryana, India). Adv. Anim. Vet. Sci. 2 (1S): 23–25.
http://dx.doi.org/10.14737/journal.aavs/2014/2.1s.23.25
Skalsky K, Yahav D, Bishara J, Pitlik S, Leibovici L, Paul M (2008). Treatment of human brucellosis: systematic review and meta–analysis of randomised controlled trials. BMJ. 336:701.
http://dx.doi.org/10.1136/bmj.39497.500903.25
PMid:18321957 PMCid:PMC2276295
Verger JM (1985). B. melitensis infection in cattle. In: Brucella melitensis, Plommet and Verger, eds. Martinus Nijhoff Publ., Dordrecht–Boston–Lancaster. 197–203.
Wright AE and Smith F (1897). On the application of the serum test to the differential diagnosis of typhoid and Malta fever. Lancet. 1: 656–9.