Advances in Animal and Veterinary Sciences
Review Article
Advances in Animal and Veterinary Sciences 2 (4): 255 – 260Infectious Causes of Gout in Chickens
Namdeo Rajendra Bulbule, Sunil Sanjay Kapgate, Milind Madhukar Chawak*,
- Poultry Diagnostic and Research Center, Division of Venkateshwara Hatcheries Private Limited, Loni–Kalbhor, Pune 412 201, India
*Corresponding author: chawakmm@rediffmail.com
ARTICLE CITATION:
Bulbule NR, Kapgate SS, Chawak MM (2014). Infectious causes of gout in chickens. Adv. Anim. Vet. Sci. 2 (4): 255 – 260.
Received: 2014–03–28, Revised: 2014–04–26, Accepted: 2014–04–26
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.4.255.260
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Gout in poultry is multifactorial in origin and can be associated to many infectious, nutritional, toxic causes or in combination that impair renal functions or cause damage kidney. The infectious agents commonly involved in the pathogenesis of gout are the nephropathogenic infectious bronchitis, and avian nephritis virus. In addition, the chicken astrovirus, found to cause gout in field and experimental cases in commercial broilers in India. The chicks up to the age of 10 days dies abruptly, gross lesions are visceral and articular gout. The pathogenicity study of the chicken astrovirus using kidney sample and virus isolates along with its molecular confirmation by RT–PCR followed by sequencing confirms that chicken astrovirus as a causative agent. Diagnosis of chicken astrovirus and avian nephritis virus from gout affected chickens can be made using degenerate RT–PCR primers targeting the polymerase gene. Reverse transcriptase polymerase chain reaction (RT–PCR) or quantitative real time PCR (qRT–PCR) are useful to differentiate avian nephritis viruses and chicken astrovirus RNA in the original tissue inoculums and also for confirmation of virus isolation. Chicken astrovirus is also important causative agent for gout or nephritis problems in addition to avian nephritis virus and infectious bronchitis virus.
INTRODUCTION
Gout is a metabolic disorder resulting in hyperuricemia and the deposition of uric acid or urates in tissues. In gout, kidney function lowers to such level which results in uric acid accumulation in the blood and body parts. It occur in two distinct forms namely, visceral and articular gout. In both the forms, deposits consist of chalky white materials called the tophi. Visceral gout is considered to be acute form and is more insidious and difficult to detect in early stages or in live birds. Clinical signs exhibited by the affected birds are not diagnostic, birds show sudden or gradual mortality. Gout is multifactorial in origin, but rather the result of kidney damage from any of a number of potential causes, which can be infectious, nutritional, toxic, poor management or possibly a combination of factors.
The nutritional or metabolic causes of gout includes excess dietary calcium, high protein diet, excess salt, low phosphorus, imbalance between Ca–P levels, adulteration of feed with urea and vitamin A and D deficiency. The prolonged dehydration can trigger urolithiasis in the birds. Water deprivation followed by over dosages of certain drugs like sulphas and the antibiotic aminoglycosides (Gentamycin) often causes gout. Feed contaminated with the mycotoxins and phytotoxins impairs kidney functions leading to gout. Citrinin, ochratoxin and oosporein are the nehrotoxic and also cause gout. Some managmental stress factors including high brooding temperature, higher level of ammonia concentration in the shed cause high mortality due to gout. Primary infectious nephritis due to bacterial agents does not appear to be common in poultry. E. coli and salmonella has occasionally been isolated from cases of pyelonephritis, but the infection is likely secondary.
The infectious causes for the development of gout are the nephropathogenic infectious bronchitis virus (IBV) and avian nephritis virus (ANV). Recently we reported the role of chicken astrovirus (CAstV) in induction of gout in commercial broilers in India. The infectious bursal disease virus also produces nephrosis or enlarged kidneys with prominent tubules containing urate deposition. The kidney lesions occur in small number of bird (about 5%) are neither constant nor specific to IBD. However, the presence of urates in kidney tubules and increase in serum uric acid could be due to kidney damage because of decreased fluid intake, diarrhea and polyuria observed in affected chicks. This review focuses on IBV and avian astrovirus infection that are involved and or associated with gout in poultry.
Economic Impact
The economic impact of astrovirus infections on the poultry industry is not fully understood (Reynolds and Schultz–Cherry, 2008). However, there is no information on the economic losses caused by CAstV to the poultry industry worldwide, astrovirus infections has been implicated in growth depression and runting–stunting syndrome which effect in financial losses due to increased culling, poor feed conversion and lower uniformity in bodyweight. Astrovirus infection in birds results primarily in mild to moderate gastroenteritis and reported to affect several different organs. In India the CAstV infections are associated with the gout particular in the commercial broilers. The outbreaks of gout causing up to 40% mortality that leads to major financial losses from high mortality with concomitant costs of treatments to the Indian broiler chicken industry.
Nephropathogenic Infectious Bronchtis Virus
Infectious bronchitis (IB) is a major health problem affecting chicken industry in most countries of the world. Infectious bronchitis virus (IBV), is an acute and highly contagious disease of chickens, characterized by respiratory distress and infection with some nephrogenic strains cause severe kidney damage, urolithisis and high mortality. Recognition of the association of infectious bronchitis virus with avian nepritis was revealed by the isolation of IBV from the kidneys of naturally infected birds. Number of factors are responsible for the severity of the nephritis associated with IBV which includes; age and breed of the birds, strain of IBV, environment and management, geographical location and immune status of the affeced flocks.
In the 1962s in United States, Winterfield and Hitchner (1962) first reported a nephrosis condition associated with IB, in the same year, Cumming (1962, 1963) reported an IB outbreak causing severe kidney lesions in chickens in Australia. Afterwards, various nephropathogenic strains of IBV have been identified around the world. In the late 1990s in Pennsylvania PA/Wolgemuth/98 and PA/171/99 nephropathogenic strains were identified (Ziegler et al., 2002) and in the early 1990s in Korea, a nephropathogenic strain designated KM91 was identified and became widespread in that country (Lee et al., 2004). The Mass (GenBank accession number HM179146) and 793B types were the most common IBV types reported in India since 1991 (Elankumaran et al., 1999). Since that time, many nephropathogenic strains of IBV associated with gout in chicks have been isolated and genetically characterized.
Bayry et al., (2005) carried out the nephropathogenic IBV isolation from the kidney samples of gout affected chickens. The disease was reported mostly in 1 to 2 weeks old broiler chicks in unvaccinated flocks. The clinical signs were gasping, upward respiration, and tracheal rales. Grossly, the birds showed distended ureters filled with uric acid and deposition of white chalky urates on other visceral organs. The microscopic kidney lesions were principally those of an interstitial nephritis, granular degeneration, vacuolation, and desquamation of tubular epithelium. Because of introduction of good vaccination programme including live IBV and inactivated nephropathogenic IBV in broiler breeder flocks, progeny were rarely affected by nephropathogenic strains of IBV in India since 2010. But in 2011, inspite of vaccination with the IBV live and inactivated nephropathogenic strain, the outbreaks of gout were observed in India.
Avian Astrovirus
Avian astrovirus infection caused severe and devastating losses to Indian poultry industry. Astrovirus infections in poultry predominantly affect young birds; however, the disease may occur in all age groups, which leads to increased susceptibility for other diseases. Avian astroviruses that were originally classified as enterovirus like viruses (McNulty et al., 1990; Todd et al., 2009) have been linked to “runting – stunting syndrome” and “growth depression” (McNulty et al., 1984; Spackman et al., 1984; Decaesstecker et al., 1986; Smyth et al., 2007). In poultry, astroviruses are more commonly recognized as a problem in turkeys, (Koci et al., 2000; Yu et al., 2000). Astroviruses in ducks have been associated with fatal hepatitis, historically known as duck hepatitis virus type II (Wool–cock and Fabricant, 1991). Astroviruses have also been isolated from broilers with poor weight gain and enteric and kidney diseases (Reynolds and Schultz–Cherry, 2008). De Wit et al., (2011) characterized a new group of avian astroviruses associated with enteric disease and locomotion problems in chickens and turkeys. Most recently, Chicken astrovirus has been isolated from gout affected chicks of commercial broilers in India (Bulbule et al., 2013).
Astroviruses have genome of 6.8–7.9 kb are small, nonenveloped RNA viruses having 28 to 30 nm in diameter. The Astroviridae family is composed of two genera, Mamastrovirus and Avastrovirus, that infect mammalian and avian species, respectively. Members of the Mamastrovirus genus include human astroviruses, which causes gastroenteritis in children, immunocompromised people, and the elderly (Glass et al., 1996). In poultry, infections with Avastrovirus genus cause disease, growth retardation (Runting and stunting syndrome), and mortality in chickens, turkeys, and ducks (Fu et al., 2009; Schultz–Cherry et al., 2000). Astrovirus causes gastroenteritis which is usually mild and self limiting in most species; however, more severe diseases have been reported in poultry such as enteritis, hepatitis and nephritis (Matsui and Greenberge, 2001).
To date, two different astrovirus species have been recognized in chickens: Avian Nephritis virus (ANV) and chicken astrovirus (CAstV). ANVs are known to cause diarrhoea, tubulonephrosis, interstitial nephritis, uricosis (gout) and, finally, death (Shirai et al., 1991). ANV was first isolated from rectal contents of the normal broiler chicks (Imada and Yamaguchi et al., 1979). ANV typically causes histological changes in the kidneys, although the viral antigen can be detected in other organs, namely liver, spleen, pancreas, jejunum and rectum (Imda et al., 1979; Shirai et al., 1992). Subclinical disease has been demonstrated by experimental infection by Jordan and Pattison, (1996). Clinical disease in chicks varies from subclinical to outbreaks of runting stunting syndrome and chick nephropathy (Frazier et al., 1990; Shirai et al., 1992; Takase et al., 1994). ANV infects only the chickens, day old chicks are more susceptible and disease has been reported up to 4 wks of age (Imada and Kawamura, 1979). ANV infection in day old chicks is associated with the severe interstitial nephritis. Narita et al., 1990 reported the increased susceptibility to ANV if the chickens are immunosuppressed. At least two distinct serotypes of ANV have been reported (Shirai et al., 1992). ANV isolates can vary with regards to pathogenicity (Shirai et al., 1992) and also for the tissue tropism (Decaesstecket et al., 1989).
Astroviruses are single–stranded, positive–sense RNA, genome containing three open reading frames which encodes: ORF1a (nonstructural protein), ORF1b (RNA–dependent RNA polymerase), and ORF2 (capsid protein) (Willcocks et al., 1994). The length varies between species and serotypes for each gene of these structures. Molecular characterization of astrovirus shows the ORF1b is the least divergent and ORF2 the most divergent among the different ORFs (Strain et al., 2008). The astrovirus capsid is responsible for attachment and entry into host cells (Sanchez–Fauquier et al., 1994; Bass et al., 1997). The capsid is a multidomain protein with a conserved N–terminal region and a highly variable C–terminal region (Jonassen et al., 2001). Chicken Astroviruses and Avian Nephritis viruses vary considerably from the mammastroviruses, although the basic character of the ORF2 amino terminus is also conserved (Mendez and arieas, 2007). Pantin–Jackwood et al., (2011) observed the variation in the capsid sequences among the astroviruses was so high that the more than 96 sequencing primers had to be designed to obtain the sequence data. The astrovirus capsid spike domain is thought to be important for host cell receptor binding, The capsid protein undergoes maturation by host extracellular proteases to become an infectious particle (Sanchez–Fauquier et al., 1994; Bass et al., 1997). Walter et al., (2001) reported that the two strains had less than 95% nucleotide homology which could be distinguished serologically. The astrovirus capsid protein has several unique biological properties such as unique inhibitors of the complement pathway by binding to complement C1 and mannose binding lectin (MBL) inhibiting the activation of the classical and lectin complement pathways, respectively (Bonaparte et al., 2008).
The isolation and characterization of CAstVs have been reported from broilers exhibiting runting syndrome and dead–in–shell chicks having hatchability problems (Spackman et al., 1984; Baxendale and Mebatsion, 2004; Smyth et al., 2009). These CAstVs are antigenically and serologically distinct from ANV, although their genomes share some sequence identity with ANV and other astroviruses. CAstV can be detected using reverse transcriptase polymerase chain reaction (RT–PCR) and quantitative (q)RTPCR targeting ORF1b and ORF2 genes (Tang et al., 2005 ; Pantin–Jackwood et al., 2006 , 2011; Todd et al., 2009 ; Smyth et al., 2010 ). Astroviruses do not grow easily in laboratory host systems and it’s difficult for serological classification. With the advent of molecular biology technique based on the production of CAstV capsid protein antigens in the insect cells using recombinant baculovirus system for the Immunofluorescence and Immunio histochemistry based diagnostic tests and for the detection of antibody using ELISA (Lee et al., 2013).
Smyth et al., (2007) detected virus–specific antigen and histological lesions in the intestine, kidney and pancreas after experimental infection of specific pathogen free (SPF) chicks with the CAstV isolate FP3. These findings show that, like ANV, CAstVs also infect internal organs. CAstVs were detected in large amounts in kidneys and intestinal contents obtained in longitudinal surveys carried out by Smyth et al., (2010) in four broiler flocks having below average performance.
However, previous studies on chicken astrovirus reported mainly their association with enteric disorders, Bulbule et al., (2013) reported the several outbreaks of gout in commercial broilers in India during 2011–2012 which caused up to 40% mortality in birds resulting in heavy economic losses to the broiler farmers and poultry industry. Laboratory investigations were carried out to find the exact cause of the gout in affected commercial broiler chicks, the Chicken Astrovirus was detected and isolated from the 80% cases of the gout, although mixed infection (ANV + CAstV) or (ANV+CAstV+IBV) were also detected from recent cases of gout in commercial broiler chicks. In the mixed infection the level of detection of CAstV was higher than for ANV and IBV. ANV or IBV alone was not detected from in these samples.
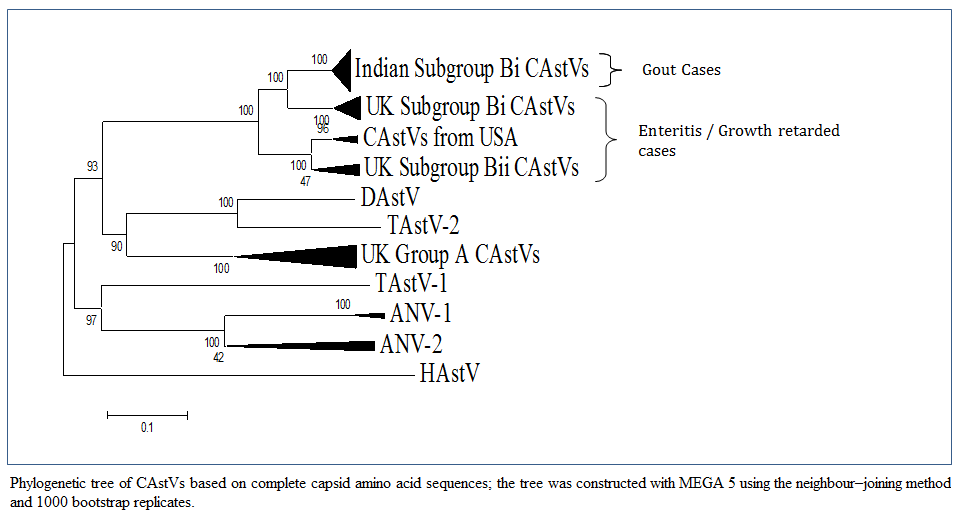
Figure 1: Phylogenetic tree of CAstVs based on complete capsid amino acid sequences; the tree was constructed with MEGA 5 using the neighbour–joining method and 1000 bootstrap replicates.
Bulbule et al., (2013) reported the case history in which one of the commercial broiler flocks experienced sudden severe mortality (35%) between 6 and 9 days of age. The major post–mortem findings were swollen kidneys, prominent ureter, visceral and articular gout. Histopathological studies revealed necrosis and degeneration of the epithelial cells of the proximal convoluted tubules with infiltration of granulocytes and interstitial lymphocytes in kidneys. Kidney samples from this particular flock were positive for CAstV and negative for IBV, ANV, chicken anaemia virus (CAV) and IBDV, as detected by qRT–PCR and qPCR and confirmed by nucleotide sequencing (Unpublished data) .
Baxandale and Mebatsion(2004) reported the isolation of the novel avian astrovirus of chickens, named CAstV is complicated by the presence of reoviruses and adenoviruses in the feaces of young chicks because these viruses grow well in CEL and LMH cell cultures and they often outgrow the more slow growing CAstV. Bulbule et al., (2013) demonstrated that the CAstV grows well in the embryonated chicken eggs through the allantoic sac route as compared to Chicken kidney cells. In the case of CAstV and ANV mixed infections, the CAstV are isolated using the allantoic route in embryonated chicken eggs from positive kidney samples (Unpublished data).
Bulbule et al., (2013) carried out the complete capsid gene sequence of Indian CAstV isolates consists of 2214 nucleotides encoding a protein of 738 amino acid residues. A phylogenetic tree analysis and pairwise comparison of the capsid protein revealed a major group of Indian subgroup Bi CAstVs (Figure 1). The CAstV detected in the UK samples can be assigned to two B subgroups and three A subgroups (Smyth et al., 2012). CAstVs belonging to the Bii subgroup were obtained from the USA (Pantin–Jackwood et al., 2011). Comparision of this Indian CAstV isolates with other avian astroviruses shows the Indian CAstV isolates and UK and USA isolates are distinct from the Duck Atsrovirus, Turkey Astrovirus, ANV–1, ANV–2 and Human Astroviruses. An intresting study on cross species transmissions probably occured during the evolutionary history of astroviruses (Van hemert et al., 2007a). Cross species transmission has also been suggested among avian species. From phylogenetic point of view DastV is more closely related to TastV2 and forms a cluster, which suggests that the duck virus may have originally transmitted from turkeys (Fu et al., 2009). Data from full genome sequencing TastV–1 and TastV–2 indicate that they are genetically unrelated and are likely to have originated from seprate introductions and should therefore be regarded as two subtypes insted of serotypes (Jonassen et al., 2003; Pantin–Jackwood et al., 2011; Strain et al., 2008).
Fundametnal Research Currently in Progress
The researches on the diversity of Chicken astroviruses circulating worldwide in the enteric and associated with concomitant infection are going out to assess the economic importance of the CAstV. Studies on the molecular characterization of CAstV based on complete capsid gene or the whole genome sequencing are going on for the study of recombination events occurred during evolution of the avastroviruses. The studies on the complete capsid or polymerase gene sequence diversity have been important implications for both the control and diagnosis of CAstV infections. Recombinant chicken astrovirus capsid protein antigen are produced using baculovirus expression system. The crystal structure studies on the astrovirus capsid surface spike domain are determined to identify three conserved patches on the surface of the spike that are candidate avian receptor–binding sites. Interspecies transmissions and a wide range of diversity of avastroviruses are identified in poultry and wild birds. These studies provide new insights into astrovirus ecology and evolution.
Diagnosis
Diagnosis of nephropathogenic IBV can be based on the clinical history, gross and histopathological lesions, IBV specific immunohistochemistry of kidney samples and virus isolation and S1 sequencing (Gelb et al., 1999; Ziegler et al., 1999). Electron microscopy is one of the principle method for the diagnosis of the Avian Astroviruses in suspected sample showing star like morphology (Madeley and Cosgrove, 1975). This method is of limited importance in some types of astrovirus including ANV and is not useful for the high sample throughput and is less sensitive (Koci et al., 2000; Tang et al., 2004; Pantin–Jackwood et al., 2007). Virus isolation in cell culture presents difficulties as astroviruses grow poorly in enteric samples (Smyth et al., 2009). But the Indian Chicken astroviruses can be isolated using the allantoic sac route in embryonated chicken eggs from SPF and also in chicken embryo kidney cells and Chicken embryo fibroblast cells (unpublished data). Bulbule et al., (2013) recommended the kidney samples from the gout affected chicks to be collected in 50% GPBS for the detection and isolation of infectious agent. Degenerate RT–PCR primers based on the avian astrovirus RNA polymerase gene designed by Tang et al., (2004) were used to amplify ANVs and CAstVs. Reverese Transcriptase polymerse chain reaction (RT–PCR) or quantitative real time PCR (qRT–PCR) are used for the initial screening and also for confirmation of virus isolates (Tang et al., 2005; Pantin–Jackwood et al ., 2006,2011; Todd et al., 2009; Smyth et al., 2010). CAstV should be included in the differential diagnosis of gout or nephritis problems in addition to ANV and IBV (Bulbule et al., 2013). Molecular characterization of avian astroviruses has been done by nucleotide sequencing of the ORF1a and ORF1b regions (Pantin–Jackwood et al., 2006; Todd et al., 2011; Bulbule et al., 2013). The capsid precursor protein gene (ORF2) is the most variable astrovirus gene, which is responsible for the variation in antigenicity and pathogenicity (Smyth et al., 2009; Pantin–Jackwood et al., 2011; Smyth et al., 2012). The complete genome sequence of Indian CAstV will be helpful for further molecular genotyping.
Current Managemetal Practices
Gout in field cases are likely to be multietiological problem and identifying a specific cause is often difficult and need the laboratory investigations. Some concerns are important for tressing out the possible cause of the gout. IBV vaccination programme to be reviewed and feed for mycotoxins and protein concentrations to be analysed. Ample quantities of fresh water are to be provided to prevent the dehydration. The electrolytes and anti–gout preparation are to be used to reduce gout mortality. Currently there is no vaccine available for the CAstV infection. The vaccination of CAstV in the breeder certainly provides the protection to hatched chicks in their early life. Along with vaccination proper biosecurity measure should reduce the infection load.
Prevention and Control
Detection and isolation of infectious bronchitis, associated with gout in IBV vaccinated birds is only the first step in control of gout in chickens. The in vivo pathogenicity of virus isolates needs to be characterized and current vaccines or combinations of commercial vaccines efficacy programme should be undertaken for the control of the gout associated with Infectious bronchitis virus. When variant virus strains are isolated from the different or particular area where the commercial vaccines do not provide adequate protection, it is necessary to develop specific vaccines to control the disease. Development of killed IBV new variant strain vaccine can be a possible tool to control the outbreak of the disease.
In the Indian context, which has suffered heavy economic losses because of gout caused by CAstV in commercial broilers, vaccination in breeders seems to be appropriate approach to prevent the gout in young commercial broiler chicks. There are currently no efficient vaccine or chemotherapeutics for the control and/or prevention of astrovirus infections (Reynolds and Schults–Cherry, 2008). Hence, strict containment is the only known method of preventing and controlling infections with any of the known astroviruses. Infected flocks, especially those that exhibit severe loss in viability and production, need to be treated with the utmost concern for biosecurity; complete sanitation of all materials and restricted access to facilities by personnel is required to contain the outbreak to the affected farm (Koci and Schultz–Cherry, 2002). To eliminate astrovirus infection, contaminated farms should be thoroughly disinfected and a minimum of 12 to 14 days down time is required. All the litter and manure should be removed and disposed off in a manner that ensures run–off does not contaminate the driveways or entrances to poultry houses. The floors, walls, fans, feeders watering systems and all equipment should then be adequately scrubbed and disinfected using compounds and procedures proven useful at eliminating highly stable small round viruses (Koci and Schultz–Cherry, 2002). Avian astroviruses are highly resistant to inactivation by disinfectants. A study by Schultz–Cherry et al., (2001) found that only formaldehyde, beta–propiolactone or peroxymonosulphate based products completely inactivates avian astrovirus. It should be recognized that chicks especially infected with CAstV are extremely susceptible to dehydration, fresh water and electrolytes should be made available to the chicks whenever possible.
Although the methods for the detection and virus isolation of the chicken astrovirus and its characterization has improved tremendously, capsid protein sequence diversity and antigenic variation exhibited by CAstVs has to be considered for the development of diagnostic reagents and for vaccine development methodologies to rapidly respond to outbreaks of the disease.
What Needs to be Done
Research and laboratory investigation support the strong link for the infectious cause of gout in commercial layers. Extensive studies on the molecular characterization of astroviruses and vaccine strain development need to be done. Current gout outbreak can be prevented by using a killed vaccine containing different CAstV isolates to vaccinate broiler breeder. This will help in raising the antibody titers in the hens and will transmit maternal antibodies to the chicks making them more resistant to the infection when they are placed on the farm.
REFERENCES
Bass DM, Upadhyayula U (1997). Characterization of human serotype 1 astrovirus–neutralizing epitopes. J. Virol. 71: 8666–8671.
PMid:9343224 PMCid:PMC192330
Baxendale W, Mebatsion T (2004). The isolation and characterization of astroviruses from chickens. Avian Pathol. 33: 364– 370.
http://dx.doi.org/10.1080/0307945042000220426
PMid:15223568
Bayry J, Goudar MS, Nighot PK, Kshirsagar SG, Ladman BS, Gelb JJr, Ghalsasi GR, Kolte GN (2005). Emergence of a nephropathogenic avian infectious bronchitis virus with a novel genotype in Indian. J. Clin. Micro. 43: 916–918.
http://dx.doi.org/10.1128/JCM.43.2.916-918.2005
http://dx.doi.org/10.1128/JCM.43.4.2039.2005
PMid:15695705 PMCid:PMC548090
Bonaparte RS, Hair PS, Banthia D, Marshall DM, Cunnion KM, Krishna NK (2008). Human astrovirus coat protein inhibits serum complement activation via C1 the first component of the classical pathway. J. Virol. 82: 817–827.
http://dx.doi.org/10.1128/JVI.01847-07
PMid:17959658 PMCid:PMC2224607
Bulbule N R, Mandakhalikar KD, Kapgate SS, Deshmukh VV, Schat KA, Chawak MM (2013). Role of chicken astrovirus as a causative agent of gout in commercial broilers in India.Avian Pathol. 42: 464–73. 6
http://dx.doi.org/10.1080/03079457.2013.828194
PMid:24015918
Cumming RB (1962). The etiology of "uremia" of chickens. Australian Vet. J. 38: 554–554.
http://dx.doi.org/10.1111/j.1751-0813.1962.tb04027.x
Cumming RB (1963). Infectious avian nephrosis (uremia) in Australia. Australian Vet. J. 39: 360–360.
http://dx.doi.org/10.1111/j.1751-0813.1963.tb04255.x
http://dx.doi.org/10.1111/j.1751-0813.1963.tb04369.x
De Wit JJ, Dam GB, de Laar JM, Biermann Y, Verstegen I, Edens F, Schrier CC (2011). Detection and characterization of a new astrovirus in chicken and turkeys with enteric and locomotion disorders. Avian Path. 40: 453–461.
http://dx.doi.org/10.1080/03079457.2011.596813
PMid:21780967
Decaesstecker M, Charlier G, Meulemans G (1986). Significance of parvoviruses enterolike viruses and reoviruses in the aetiology of the chicken malabsorption syndrome. Avian Pathol. 15: 769 – 782.
http://dx.doi.org/10.1080/03079458608436339
PMid:18766578
Elankumaran S, Balachandran C, Chandran ND, Roy P, Albert A, Manickam R (1999). Serological evidence for a 793B related avian infectious bronchitis virus in Indian. Vet. Rec.144: 299–300.
PMid:10204229
Frazier JA, Howes K, Reece RL, Kidd AW, Cavanagh D (1990). Isolation of noncytopathic viruses implicated in the aetiology of nephritis and baby chick nephropathy and serologically related to avian nephritis virus. Avian Pathol. 19: 139–160.
http://dx.doi.org/10.1080/03079459008418663
PMid:18679921
Fu Y, Pan M, Wang X, Xu Y, Xie X, Knowles NJ, Yang H, Zhang D (2009). Complete sequence of a duck astrovirus associated with fatal hepatitis in ducklings. J. Gen. Virol. 90: 1104–1108.
http://dx.doi.org/10.1099/vir.0.008599-0
PMid:19264607
Gelb JJ, Ladmanm BS, Nix WA, Pope CR (1999). Chracteristics of nephropathogenic infectious bronchitis virus in Pennsylvania Poultry. JAVMA 215: 1678
Glass RI, Noel J, Mitchell D, Herrmann JE, Blacklow NR, Pickering LK, Dennehy P, Ruiz–Palacios G, de Guerrero ML, Monroe SS (1996). The changing epidemiology of astrovirus–associated gastroenteritis: a review. Arch.Virol. 12: 287–300.
Imada T, Yamaguchi S, Kawamura H (1979). Pathogenicity for baby chicks of the G–4260 strain of the picornavirus "avian nephritis virus." Avian Dis. 23: 582–588.
http://dx.doi.org/10.2307/1589733
PMid:230802
Jonassen CM, Jonassen TO, Saif YM, Snodgrass DR, Ushijima H, Shimizu M, Grinde B (2001). Comparison of capsid sequences from human and animal astroviruses. J. Gen. Virol 82: 1061–1067.
PMid:11297680
Jonassen CM, Jonassen TT, Sveen TM, Grinde B (2003). Complete genomic sequences of astroviruses from sheep and turkey: comparison with related viruses. Virus Res.91: 195–201.
http://dx.doi.org/10.1016/S0168-1702(02)00269-1
Koci MD, Seal BS, Schultz–Cherry S (2000). Molecular characterization of an avian astrovirus. J. Virol. 74: 6173–6177.
http://dx.doi.org/10.1128/JVI.74.13.6173-6177.2000
PMid:10846102 PMCid:PMC112117
Koci MD, Schultz–Cherry S (2002). Avian astroviruses. Avian Pathol. 31: 213–227.
http://dx.doi.org/10.1080/03079450220136521
PMid:12396344
Lee A, Wylie M, Smyth VJ, Skibinska A, Patterson IA, Forster F, Welsh MD, Todd D (2013). Chicken astrovirus capsid proteins produced by recombinant baculoviruses: potential use for diagnosis and vaccination.Avian Pathol. 42(5):434 -442.
http://dx.doi.org/10.1080/03079457.2013.822467
PMid:24066895
Lee SK, Sung HW, Kwon HM (2004). S1 glycoprotein gene analysis of infectious bronchitis viruses isolated in Korea. Arch. Virol. 149: 481–494.
http://dx.doi.org/10.1007/s00705-003-0225-3
PMid:14991438
Lee SK, Sung HW, Kwon HM (2004). S1 glycoprotein gene analysis of infectious bronchitis viruses isolated in Korea. Arch. Virol. 149:481–494.
http://dx.doi.org/10.1007/s00705-003-0225-3
PMid:14991438
Madeley CR, Cosgrove BP (1975). Letter:28nm particles in faeces in infantile gastroenteritis. Lancet. 2: 451–452.
http://dx.doi.org/10.1016/S0140-6736(75)90858-2
Matsui SM, Greenberg HB (2001). Astrovirus. In D.M. Knipe and P.M. Howley (Eds.), Fields Virology 4th edn, vol 1, (pp. 875–893). Baltimore, MD: Lippincott Williams and Wilkins.
McNeilly F, Connor TJ, Calvert VM, Smyth JA, Curran WL, Morley AJ, Thompson D, Singh S, McFerran JB, Adair BM, McNulty MS (1994). Studies on a new enterovirus–like virus isolated from chickens. Avian Pathol. 23: 313– 327.
http://dx.doi.org/10.1080/03079459408418999
PMid:18671096
McNulty MS, Allan GM, Connor TJ, McFerran JB, McCracken RM (1984). An entero–like virus associated with the runting syndro me in broiler chickens. Avian Pathol. 13: 429 – 439.
http://dx.doi.org/10.1080/03079458408418545
PMid:18766858
McNulty MS, Connor TJ, McNeilly F, McFerran JB (1990). Biological characterisation of avian enteroviruses and enterovirus–like viruses. Avian Pathol. 19: 75 – 87.
http://dx.doi.org/10.1080/03079459008418658
PMid:18679916
Mendez E, Arias CF (2007). Astroviruses. In D.M. Knipe, P.M. Howley, D.E. Griffin, R.A. Lamb, S.E. Straus, M.A. Martin, & B. Roizman (Eds), Fields Virology, Vol.1, 5th edn (pp 981–1000). Philadelphia: Lippincott–Williams and Wilkins.
Pantin–Jackwood MJ, Spackman E, Day JM, Rives D (2007). Periodic monitoring of commercial turkeys for enteric viruses indicates continuous presence of astrovirus and rotavirus on the farms. Avian Dis. 51: 674–80
http://dx.doi.org/10.1637/0005-2086(2007)51[674:PMOCTF]2.0.CO;2
http://dx.doi.org/10.1637/1933-5334(2007)2[e4:PMOCTF]2.0.CO;2
Pantin–Jackwood MJ, Spackman E, Woolcock PR (2006). Molecular characterization and typing of chicken and turkey astro viruses circulating in the United States: implications for diagnostics. Avian Dis. 50: 397–404.
http://dx.doi.org/10.1637/7512-020606R.1
PMid:17039840
Pantin–Jackwood MJ, Strother KO, Mundt E, Zsak L, Day JM, Spackman E (2011). Molecular characterization of avian astroviruses. Arch. Virol.156: 235– 244.
http://dx.doi.org/10.1007/s00705-010-0849-z
PMid:21069394
Reynolds DL, Schultz–Cherry S (2008). Astrovirus infections. In Y. M. Saif A.M. Fadly J.R. G lisson L.R. McDougald L.K. Nolan and D.E. Swayne (Eds.). Diseases of Poultry 12th edn Ames IA: Blackwell Publishing.
Sanchez–Fauquier A, Carrascosa AL, Carrascosa JL, Otero A, Glass RI, Lopez JA, San Martin C, Melero JA (1994). Characterization of a human astrovirus serotype 2 structural protein (VP26) that contains an epitope involved in virus neutralization. Virol. 201: 312–320.
http://dx.doi.org/10.1006/viro.1994.1296
PMid:7514320
Schultz–Cherry S, Kapczynski DR, Simmons VM, Koci MD, Brown C, Barnes HJ (2000). Identifying agent(s) associated with poult enteritis mortality syndrome: importance of the thymus. Avian Dis. 44: 256–265.
http://dx.doi.org/10.2307/1592538
PMid:10879904
Shirai J, Nakamura K, Nozaki H, Kawamura H (1991). Differences in the induction of urate deposition of specific pathogen–free chicks inoculated with avian nephritis virus passaged by five different methods. Avian Pathol. 35: 269 – 275.
Shirai J, Tanimura N, Uramoto K, Narita M, Nakamura K, Kawamura H (1992). Pathologically and serologically different avian nephritis virus isolates implicated in etiology of baby chick nephropathy. Avian Dis. 36: 369 – 377.
http://dx.doi.org/10.2307/1591515
PMid:1320866
Smyth JA, Connor TJ, McNeilly F, Moffet DA, Calvert VM, McNulty MS (2007). Studies on the pathogenicity of enterovirus–like viruses in chickens. Avian Pathol. 36: 11 9 – 126.
Smyth VJ, Jewhurst HL, Adair BM, Todd D (2009). Detection of chicken astrovirus by reverse tra nscriptase–polymerase chain reaction. Avian Pathol. 38: 293 – 299.
http://dx.doi.org/10.1080/03079450903055397
PMid:19937514
Smyth VJ, Jewhurst HL, Wilkinson DS, Adair BM, Gordon AW, Todd D (2010). Development and evaluation of real–time TaqMan RT–PCR assays for the detection of avian nephritis virus and chicken astrovirus in chic kens. Avian Pathol. 39: 467 – 474.
http://dx.doi.org/10.1080/03079457.2010.516387
PMid:21154056
Smyth VJ, Todd D, Trudgett J, Lee A, Welsh MD (2012). Capsid protein sequence diversity of chicken astrovirus. Avian Pathol. 41: 151 – 159.
http://dx.doi.org/10.1080/03079457.2011.652938
PMid:22515534
Spackman D, Gough RE, Collins MS, Lanning D (1984). Isolation of an enterovirus–like agent in the meconium of dead–in–shell chicken embryos. Vet. Rec. 114: 216– 218.
http://dx.doi.org/10.1136/vr.114.9.216-a
PMid:6328734
Strain E, Kelley LA, Schultz–Cherry S, Muse SV, Koci MD (2008). Genomic analysis of closely related astroviruses. J. Virol. 82: 5099–5103.
http://dx.doi.org/10.1128/JVI.01993-07
PMid:18321976 PMCid:PMC2346738
Takase K, Uchimura T, Yamamoto M, Yamada S (1994). Susceptibility of embryos and chicks derived from immunized hens to avian nephritis virus. Avian Pathol. 23: 117–125
http://dx.doi.org/10.1080/03079459408418979
PMid:18671076
Tang Y, Ismail MM, Saif YM (2005). Development of antigen captures enzyme–linked immunosorbent assay and RT–PCR for detecti on of turkey astroviruses . Avian Dis. 49: 182 – 188.
http://dx.doi.org/10.1637/7255-080504R
PMid:16094820
Todd D, Smyth VJ, Ball NW, Donnelly BM, Wylie M, Knowles NJ, Adair BM (2009) Identification of chicken enterovirus–like viruses duck hepatitis virus type 2 and duck hepatitis virus type 3 a s astroviruses. Avian Pathol. 38: 21 – 30.
http://dx.doi.org/10.1080/03079450802632056
PMid:19156577
Todd D, Trudgett JS, Smyth VJ, Donnelly B, McBride N, Welsh MD (2011). Capsid protein sequence diversity of avian nephritis virus. Avian Pathol. 40: 249 – 259.
http://dx.doi.org/10.1080/03079457.2011.553583
PMid:21711184
Van Hemert FJ, Lukashov VV, Berkhout B (2007). Different rates of (non–)synonymous mutations in astrovirus genes; correlation with gene function. Virology 4: 25.
http://dx.doi.org/10.1186/1743-422X-4-25
PMid:17343744 PMCid:PMC1828050
Walter JE, Mitchel DK, Guerrero ML, Berke T, Matson DO, Monroe SS, Pickering LK, Ruiz–Palacios G (2001). Molecular epidemiology of human astrovirus diarrhea among children from a periurban community of mexico city. J. Infec. Dis. 183:681–686.
http://dx.doi.org/10.1086/318825
PMid:11181143
Willcocks MM, Brown TD, Madeley CR, Carter MJ (1994). The complete sequence of a human astrovirus. J. Gen. Virol 75: 1785–1788.
http://dx.doi.org/10.1099/0022-1317-75-7-1785
PMid:8021608
Winterfield RW, Hitchner SB (1962). Etiology of an infectious nephritis–nephrosis
Woolcock PR, Fabricant J (1991). Duck virus hepatitis. In B.W. Calneck (Ed.) Diseases of Poultry. Ames IA: Iowa State University Press.
PMCid:PMC1670311
Yu M, Ismail MM, Qureshi MA, Dearth RN, Barnes HJ, Saif YM (2000). Viral agents associated with poult enteritis and mortality syndrome: the role of a small round virus and a turkey coronavirus.Avian Dis. 44: 297–304.
http://dx.doi.org/10.2307/1593099
http://dx.doi.org/10.2307/1592543
PMid:10879909
Ziegler AF, Dunn PA, Gelb J, Weinstock D, Castro M, Davison M (1999). Nephropathogenic infectious bronchitis virus infections in Pennsylvania poultry. JAVMA 215: 1689
Ziegler AF, Ladman BS, Dunn PA, Schneider A, Davison S, Miller PG, Lu H, Weinstock D, Salem M, Eckroade RJ, Gelb JJ (2002). Nephropathogenic infectious bronchitis in Pennsylvania chickens 1997– 2000. Avian Dis. 46: 847–858.
http://dx.doi.org/10.1637/0005-2086(2002)046[0847:NIBIPC]2.0.CO;2




