Advances in Animal and Veterinary Sciences
Case Report
Serum Biochemistry Profiles in Confirmed Effusive Feline Infectious Peritonitis Cats
Alfarisa Nururrozi1*, Dhasia Ramandani2, Madarina Wasissa3, Yanuartono1, Soedarmanto Indarjulianto1
1Department of Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada; 2Department of Bioresource and Veterinary Technology, Vocational College, Universitas Gadjah Mada; 3Postgraduate Student, Veterinary Science, Faculty of Veterinary Medicine, Universitas Gadjah Mada.
Abstract | Feline infectious peritonitis (FIP) is one of the most challenging diseases due to difficulties in treatment. A reliable and fast diagnosis is crucial to the best prognosis. The aim of this study was to evaluate the serum biochemistry profile of cats with confirmed effusive FIP. A total of 32 cats suspected of showing clinical symptoms at Animal Clinic, Faculty Veterinary Medicine, Universitas Gadjah Mada were used in this study. Ten serum biochemistry from cats who had been diagnosed with effusive FIP were analyzed. The diagnosis was based on clinical examination, ultrasound, rivalta test, and PCR. The serum chemistry profiles showed the average levels of SGPT and SGOT in effusive FIP were increased 132.2±36.3 IU/L and 103.3±48.7 IU/L respectively (P<0.05). The average albumin has normal range of 2.3±0.1 g/dL. Hyperglobulinemia was found in 90% of samples with an average of 6.8±0.4 g/dL (P<0.05). All cats have low albumin-globulin ratio with an average of 0.3±0.2 (P<0.05). The serum biochemistry profiles in effusive FIP cats including increased levels of SGPT and SGOT, hypoalbuminemia, hyperglobulinemia, and the decreased ratio albumin-globulin.
Keywords | Cat, FIP, Effusive, Serum
Received | August 04, 2021; Accepted | October 24, 2021; Published | December 01, 2021
*Correspondence | Alfarisa Nururrozi, Department of Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada; Email: alfarisa.nururrozi@ugm.ac.id
Citation | Nururrozi A, Ramandani D, Wasissa M, Yanuartono, Indarjulianto S (2022). Serum biochemistry profiles in confirmed effusive feline infectious peritonitis cats. Adv. Anim. Vet. Sci. 10(1): 126-130.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.1.126.130
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2022 Nururrozi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Feline Infectious Peritonitis (FIP) is a fatal disease in cats caused by Feline Coronavirus (FCoV) of the Coronaviridae family (Addie et al., 2009). Manifestations of FIP cases are divided into dry and effusive types (Kipar and Meli, 2014). Symptoms of the effusive type are characterized by the accumulation of fluid in the abdominal (Pedersen et al., 2015). This disease is high mortality and has no effective treatment, so a fast and reliable diagnosis is crucial for prognostic purposes (Addie et al., 2015).
Several methods to detect FCoV infection have been reported previously including molecular detection using RT-PCR and immune-staining as the gold standard method (Kipar and Meli, 2014; Sharif et al., 2010). Examination of effusion fluid using immunofluorescent staining is very specific, but the procedure is complex and depends on the number of macrophages in the effusion fluid (Giori et al., 2011). The diagnosis of FIP at the clinical level has not been encouraging. The difficulty of the FIP diagnosis methods is due to the absence of pathognomonic clinical symptoms, the low sensitivity and specificity of the rapid test (Hartmann, 2005; Addie et al., 2015; Stranieri et al., 2018; Felten and Hartmann, 2019).
One of the most common diagnostic tests performed by veterinarians is a hematology examination. Currently, hematological analyzes were relatively the most feasible approach for the diagnosis in Indonesia’s veterinary clinic based on affordable prices and testing facilities. The difference with research by Riemer et al. (2016), this study only used samples from the effusive type while previous studies used both types of FIP. The two types of FIP have different clinical features and pathogenesis, although there is considerable overlap between the two forms. The presence of effusion is the first consideration for further analysis because it is relatively non-invasive and is very useful for differentiating types of FIP. Due to the different prognoses, it is important to determine whether the FIP is effusive or non-effusive. This study aims to evaluate the serum biochemistry profile of cats with confirmed effusive FIP.
Case presentation
Samples
A total of 32 cats suspected of showing clinical symptoms were used in this study. Then, ten samples (range 6 months – 5 years of age) with confirmed effusive FIP were analyzed. Effusive FIP was confirmed based on the results of clinical examination, ultrasound examination, rivalta test, and PCR. Only patients showing positive results in all these tests, had their blood chemistry profiles analyzed. Cats diagnosed with FIP but not showing abdominal or thoracic effusion are ruled out. All samples were collected from the Animal Clinic, Faculty of Veterinary Medicine, Gadjah Mada University. The serum samples were collected before any treatment. The data collected related to the patient was under the owner’s consent.
Clinical findings
Clinical examination was carried out by inspection, palpation, and auscultation methods. The cat used for this study showed an effusive FIP characterized by the presence of peritonitis, pleuritis, or pericarditis with effusion in the abdomen and thorax (Figure 1).
Ultrasound examination
Ultrasound examination was performed by SG-10 (Sogata, China) using a transducer frequency of 5-13 MHz. The cat used in this study had ultrasound examination results that lead to effusive FIP characterized by ascites, and effusion in the thorax (Tsai et al., 2011). The results of the ultrasound examination of the abdominal cavity are shown in Figure 2.
Rivalta test
Rivalta test is performed to differentiate transudate and exudate in effusion fluid. Peritoneal fluid was collected by the abdominocynthesis method. A total of 0,5 ml of ascites was tested on a mixture of 98% glacial acetic acid and 8 ml of distilled water. The typical fluid from FIP cases is viscous, yellowish in color, clear or cloudy, and usually forms lumps due to its high protein content (Fischer et al., 2012). All cats diagnosed with effusive FIP showed positive results in the rival test.
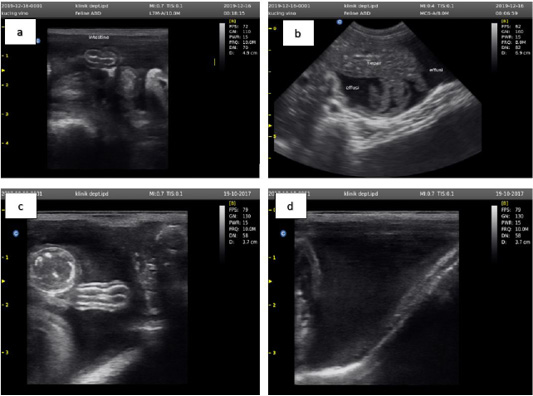
Figure 2: Ultrasound examination results showed an accumulation of anechoic fluid between the small intestine (a), liver (b), large intestine (c), and outside the bladder wall (d).
Polymerase chain reaction
PCR was performed using the lubs primer and specific forward primers designed to be specific for FCoV (Iffs) described by Addie et al. (2003). The sequences of the primer sets are as follows: forward primer, 5′-GTTTCAACCTAGAAAGCCTCAGAT-3’. The size of amplified DNA fragments using these primers was expected to be 376 bp.
Blood chemistry examination
The blood samples were collected from the femoral vein of each cat. The serum biochemistry examination to determine serum levels of glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), albumin, globulin, and the ratio between albumin and globulin. The SGPT and SGOT examination methods were carried out with a portable photometer (Microlab 300 LX, Elitechgroup USA), while the albumin and globulin measurement method with the photometer principle (Photometer 5010, Riele Germany).
Data analysis
The statistical analysis of the data was carried out by T-test with the SPSS software 16.0. (IBM Corporation, USA). P < 0.05 was considered significant for all tests. The results of the blood chemistry examination are shown in Table 1.
Table 1: Results of effusive FIP cat blood chemistry (n=10).
| Parameter | Average (n=10) | Reference* | P-value | Increase samples (%) | Normal samples (%) | Decrease samples (%) |
| SGPT (lU/L) | 132.2±36.3 | 28-76 |
0.017 a |
60 | 40 | 0 |
| SGOT (lU/L) | 103.3±48.7 | 5-55 |
0.005 a |
50 | 50 | 0 |
| Albumin (g/dL) | 2.3±1.6 | 2.4-4.1 | 0.844 | 0 | 40 | 60 |
| Globulin (g/dL) | 6.8±0.4 | 2.6-5.1 |
0.003 a |
90 | 10 | 0 |
| Ratio Albumin Globulin | 0.3±0.2 | 0.8 |
0.008 a |
0 | 0 | 100 |
| Rivalta | Positive | Positive | - | - | - |
*Reference: Schalm, 2010. a Indicates a significant difference.
A significant increase in SGPT and SGOT was observed in effusive FIP cats (P < 0.05). The average of SGPT and SGOT levels have increased two-fold from the normal range. The average levels of SGPT and SGOT in effusive FIP were 132.2±36.3 IU/L and 103.3±48.7 IU/L respectively. This average increased from the normal SGPT and SGOT values. SGPT and SGOT measurements were used to detect liver cell damage. SGPT is a specific enzyme for the detection of liver disease, while SGOT is not a specific enzyme because it can come also from muscles. The Increased levels of SGOT and SGPT possibly occur due to changes in plasma membrane permeability in liver cells. This condition is due to inflammation of the liver as a result of advanced peritonitis (Tsai et al., 2011; Malbon et al., 2019).
The results of serum albumin examination showed the average albumin level was slightly below the normal range (P > 0.05). However, 60% of the sample showed hypoalbuminemia. Low levels of serum albumin occur due to decreased production in the liver by FIP infection. Furthermore, decreased albumin production in the liver will causes disturbances in plasma oncotic pressure. As a result, disturbance of plasma oncotic pressure cause a loss of protein fluid, which will cause the albumin value to continue to decline.
The most consistent serum biochemistry finding in effusive FIP is an increase of globulin (P < 0.05). A total of 90% had hyperglobulinemia while 10% of the samples were within the normal range. The average result of globulin in effusive FIP was 6.8 ± 0.4 g/dL. These results increased than normal globulin values (2.6-5.1 g/dL). Research by Riemer et al. (2016), stated that 89.1% of FIP cats (163/183) regardless of effusion or not, had hyperglobulinemia. High levels of serum globulin can occur due to the specific anti-FCoV immune response. According to Paltrinieri et al. (2002), hyperglobulinemia has a positive correlation with antibody titers in FIP cases. Increased levels of these globulins will cause blood protein levels also increase.
The average albumin-globulin ratio (A:G) on FIP cats was significantly decreased compared with the literature (P < 0.05). According to Addie et al. (2009), the A:G ratio test in FIP cases has a higher diagnostic value than routine hematological tests. Similarly, the opinion of Felten and Hartmann (2019) also stated that the A:G ratio has a more diagnostic approach than gamma-globulins or total protein. The opinions of other researchers classify the results of the A:G ratio as potential FIP if the value is <0.4 and not FIP if >0.6-0.8 (Tsai et al., 2011; Jeffery et al., 2012). Hartmann (2005) explained the serum albumin-to-globulin ratio is less than 0.8, the probability of the cat has FIP is high (92% positive predictive value).
The A:G ratio can fluctuate depending on the condition of each cat. Some researchers argue that an increase of globulin may cause negative feedback on albumin production in the liver (Hartmann, 2005). Other disease conditions such as chronic respiratory disease and chronic stomatitis can cause an increase in globulins and plasma proteins, which can obscure the diagnosis of FIP based on A:G ratio. Individual immune each cat as a responses to produce albumin and antibodies also different, which will cause the A:G ratio to fluctuate.
The results of this study showed the blood chemical profile in FIP effusion cats were increased levels of SGPT and SGOT, hypoalbuminemia, hyperglobulinemia, and the decreased ratio between albumin and globulin. The hemogram profile was similar to the previous study by Paltrinieri et al. (2002), Jeffery et al. (2012), Riemer et al. (2016), and Felten and Hartmann (2019). Based on Riemer et al. (2016) cats diagnosed with effusive FIP had non-regenerative anemia, leukocytosis with neutrophilia and lymphopenia, hypoalbuminemia, hyperglobulinemia, decreased A:G ratio, and increased concentrations of 2-β-ɣ-globulin.
According to Hartmann (2005), a low albumin-globulin ratio (less than 0.8) is a good diagnostic approach for FIP effusion with a positive predictive value (PPV) of 92%. Furthermore, according to Felten and Hartmann (2019), hyperglobulinemia also has a significant hematological approach for FIP with 99% specificity, 35% sensitivity, and 98% PPV. Although the researchers stated leukocytosis with lymphopenia and neutrophilia is a typical hemogram in effusive FIP, it also is interpreted as a typical “stress leukogram” caused by various other systemic diseases (Addie et al., 2015).
This hemogram profile can occur in various conditions, but researchers have come to the consensus that none of the changes found are pathognomonic or specific only to effusive FIP. This hemogram profile can also occur in various other diseases, which is the differential diagnosis of FIP. Therefore, the diagnosis needs to be made based on molecular detection using RT-PCR and immune-staining. This research uses a limited number of samples, which may affect the final results of the study. The addition of the number of samples and the grouping of infected cat signals (age and sex) are necessary for further research. In the future, the larger number of samples hopefully can explain more comprehensively the profile of serum biochemistry in effusive FIP.
CONCLUSIONS AND RECOMMENDATIONS
The serum biochemical profile in FIP effusion cats including increased levels of SGPT and SGOT, hypoalbuminemia, hyperglobulinemia, and the decreased ratio albumin- globulin.
ACKNOWLEDGEMENTS
The authors are highly thankful to the Department of Internal Medicine, Faculty Veterinary Medicine, Universitas Gadjah Mada for the support this research.
Novelty Statement
The novelty of this study provides preliminary information on biochemistry profiles in the case of FIP. The findings will help veterinary services in Indonesia in the effort to diagnose FIP. Further investigation with a larger number of samples were required to provide a more complete biochemistry profile.
Author’s Contribution
All authors contributed equally to the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






