Advances in Animal and Veterinary Sciences
Research Article
Evaluation of Sperm DNA Fragmentation using TUNEL Assay in Different Animal Species
Kurniawan Dwi Prihantoko1, Makruf Arif1, Asmarani Kusumawati2*, Diah Tri Widayati3, Agung Budiyanto2
1Postgraduate of Veterinary Science, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Jl. Fauna No. 2, Karangmalang, Sleman, Yogyakarta 55281, Indonesia; 2Department of Reproduction and Obstetrics, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Jl. Fauna No. 2, Karangmalang, Sleman, Yogyakarta 55281, Indonesia; 3Department of Animal Breeding and Reproduction, Faculty of Animal Science, Universitas Gadjah Mada, Jl. Fauna No. 2, Karangmalang, Sleman, Yogyakarta 55281, Indonesia.
Abstract | The studies of spermatozoa DNA fragmentation at the level of different animal species are very limited. This study examined the sperm DNA fragmentation in several animal species (bulls, chickens, and mice) by utilizing terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. This study used a total of 24 different semen samples of Peranakan Ongole (PO bulls) or Ongole grade bull, chickens (KUB Chicken), and mice. Eight semen samples each were collected by three different methods. Semen samples of Peranakan Ongole (PO) bull were collected by artificial vagina method, semen samples of Kampung Unggul Balitnak (KUB) chicken were collected by abdominal massage method, and semen samples of mice were collected by epididymal collection method. Evaluation rate included the motility (%), viability (%) by eosin-nigrosine staining method, membrane integrity (%) by hypo-osmotic swelling test (HOST) method and sperm DNA fragmentation (%) by the TUNEL assay method. The results showed mice had the highest DNA fragmentation rates as compared to others species under study, while bull semen samples showed the lowest rates of DNA fragmentation. Significant differences in mice semen could be affected by the quality of chromatin. The TUNEL method might not effectively interpret the chicken semen samples better than the bulls and mice. However, sperm DNA fragmentation can be used to determine semen quality comprehensively based on the rates of DNA damage.
Keywords | DNA fragmentation, Semen quality, Semen evaluation, Sperm, TUNEL
Received | June 14, 2021; Accepted | September 19, 2021; Published | December 01, 2021
*Correspondence | Asmarani Kusumawati, Department of Reproduction and Obstetrics, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Jl. Fauna No. 2, Karangmalang, Sleman, Yogyakarta 55281, Indonesia; Email: uma_vet@ugm.ac.id
Citation | Prihantoko KD, Arif M, Kusumawati A, Widayati DT, Budiyanto A (2022). Evaluation of sperm DNA fragmentation using TUNEL assay in different animal species. Adv. Anim. Vet. Sci. 10(1): 14-19.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.1.14.19
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2022 Prihantoko et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Frozen semen is an essential technology for artificial insemination or livestock resource development (Gliozzia et al., 2011). One of the most crucial aspects of cryopreservation is the quality of the semen itself. The sperm quality is evaluated by microscope characteristics evaluation, such as motility and viability (Venkatesh et al., 2011). The motility rates and individual motion serve as the fertilization indicator capacity, though it is unable to accurately predict the fertilization ability (Prihantoko et al., 2020a). A deep structure and mechanism evaluation of sperm may lead to a better understanding to predict both the semen capacity and ability to fertilize and resist cryopreservation. In the conventional assessment of semen quality, DNA status is not assessed even though the DNA integrity is essential in preventing failures in the fertilization process and embryo development (Gliozzia et al., 2011). However, as in humans, it is impossible to accurately assess the sperm by conventional method in predicting the male reproductive succession to AI (Ming-Wen and Lloyd, 2020). Normal embryonic development depends on the sperms which can carry intact DNA (Aitken et al., 2013; Barratt, 2010). In both human and animal semen, DNA damage is caused by multiple factors, such as a lapse in spermiogenesis, weak chromatin compaction, sperm apoptosis, oxidative stress, drug agents, radiations, infections, and others (Sakkas and Alvarez, 2010).
The sperm DNA damage is an essential parameter to evaluate the semen before animal-assisted reproductive technology (ART) such as in-vitro fertilization (IVF) and artificial insemination (AI) is used (Takeda et al., 2015). DNA damage may cause failure in pregnancy and obstruct the artificial insemination process (Singh and Agarwal, 2011). However, the specific mechanism of DNA damage affecting male fertility remains unclear (Zini et al., 2011). The single-strand or double-strand breaks, deletions, and additions, or base modifications are involved in DNA damage (Takeda et al., 2015). Strand break in sperm DNA may be caused by reactive oxygen species (ROS) (Irvine et al., 2000). According to Agarwal and Allamaneni (2005), the single-cell gel electrophoresis (Comet assay) and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) are the common methods for DNA fragmentation. The disruption of the DNA strand primarily caused by ROS (Tremellen, 2008) may be detected using the TUNEL assay (Takeda et al., 2015). The previous studies concluded that TUNEL assay is the most effective method to detect sperm DNA damage (Sharma et al., 2010, 2013; Takeda et al., 2015). Other studies conducted with different methods had shown a negative correlation between fertility rates and genetic material quality (Frazer, 2004; Gliozzia et al., 2011; Mahmoud et al.,2015). In humans, infertility is affected by higher levels of DNA damage (Irvine et al., 2000). The infertile sperm is found to be more susceptible to DNA damage (Gliozzia et al., 2011). Besides, the high percentage of DNA damage showed significantly lower conception rates, as detected by the TUNEL assay (Henkel et al., 2004). Further, it was found in a study that the semen percentage of TUNEL-positive can determine the infertile men in the fertile group (Sharma et al., 2010).
The studies of sperm DNA integrity that have been carried out in humans (Donnelly et al., 2001) differed from those of bovine and birds in which the issue is not commonly studied (Madeddu et al., 2009). The sperm DNA integrity is commonly been studied in human and mice samples. The present study examined the sperm DNA fragmentation in bovine, chicken, and mice by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay and correlation between sperm DNA damage and conventional semen quality parameters.
MATERIALS AND METHODS
Semen collection and evaluation
This study used a total of 24 different semen samples of Peranakan Ongole bulls (Ongole grade bull), chickens (KUB Chicken), and mice, eight ejaculated semen samples each were collected from those three different species. Semen samples of Peranakan Ongole (PO) bull were collected by artificial vagina method from eight different Indonesian Ongole grade bulls aged 4 to 6 years old in health conditions with normal reproductive organs. The bulls were treated in Balai Pengembangan Bibit, Pakan Ternak dan Diagnostik Kehewanan Yogyakarta (Yogyakarta Animal Breeding Center, Indonesia). KUB chicken semen samples were collected by abdominal massage method at the field laboratory, Faculty of Veterinary Medicine, Gadjah Mada University, Indonesia. mice semen samples were collected by epididymal collection method at the Laboratory of Obstetrics and Gynecology, Faculty of Veterinary Medicine, Gadjah Mada University, Indonesia. The spermatozoa evaluation parameters include motility (%), viability (%), membrane integrity (%), and DNA sperm fragmentation (%).
Motility of spermatozoa
Sperm motility evaluation was subjectively assessed under a microscope. A drop of semen well-mixed in four drops of saline solution was placed on an object glass and covered with a coverslip. Sperm motility was being evaluated under 40 x magnification in five different fields of the object glass. Then the evaluation was assessed in percentages on a scale range of 0-100%.
Viability of spermatozoa
Sperm viability was assessed by the eosin-nigrosin staining method (Telnoni et al., 2017). The procedure started by mixing 10 µL semen with 20 µL eosin-nigrosin (1:2) on an object glass. Followed by smear preparation was later using another object glass and fixation with a bunsen burner. Semen viability was later evaluated using a microscope under 40 magnification. Sperm viability were calculated by comparing the number of live and dead spermatozoa from a total of 200 sperm cells. An intact membrane of live sperm would prevent its staining, but the dead sperm showed otherwise.
Membrane integrity of spermatozoa
The sperm membrane integrity was assessed by hypo-osmotic swelling test (HOST) according to Akcay et al. (2012) method with a slight modification. 10 µL of semen diluted with 100 µL of HOST solution (a mixture of 0.9 fructose, 0.49 g of citrate sodium, and distilled water to a final volume of 100 ml) and incubated for 30 minutes at 37 oC. The solution was then smeared on the object glass, dried, and fixed. The membrane integrity rates were assessed by comparing the number of intact membrane sperm and the damaged membrane spermatozoa. The circular tail spermatozoa represent an intact membrane, but a damaged membrane showed otherwise with a straight tail.
Sperm DNA fragmentation
Sperm DNA fragmentation was examined by using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay or in-situ Cell Death Detection Kit, TMR Red version 12th (Sigma-Aldrich, USA). The samples of semen were smeared to an object glass, dried, and fixated for an hour at 15-25oC followed by rinsed with phosphate buffer saline (PBS). The stained samples were added 0.1% Triton X-100 in sodium citrate 0.1% for two minutes at 2-8oC and rinsed with PBS twice. The negative control was made by adding 50 µL label solution and the positive control was made by incubating the samples with DNAse recombinant to induct DNA separation. The stained samples and controls were dried and mixed with the TUNEL assay reaction available in the kit at 37oC for 60 minutes and rinsed with PBS three times. The results were examined using a laser-scanning confocal microscope at a wavelength of 517 nm. Spermatozoa in a bright green fluorescence showed damaged (fragmented) DNA, while spermatozoa in a dull green fluorescence showed normal DNA.
Statistical analysis
Analytical descriptive and coefficient corelative were performed by SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) as the analysis tool. Duncan’s multiple range test was used for treatment comparisons.
RESULTS AND DISCUSSIONS
TUNEL assay is the most used method to evaluate DNA fragmentation. The TUNEL assay kits, relatively well-developed and cost-effective, are mass-produced. Also, it is simple to test TUNEL-positive spermatozoa through fluorescence microscope or flow cytometry (Sharma et al., 2013). We analyzed the semen samples through a microscope to further evaluate relations between DNA damage to the conventional semen quality parameters. Results of this study (Table 1, Figure 1) showed that motility rates in KUB chickens were the highest among others, and there was a significant difference in the semen motility rates of Peranakan Ongole breed bulls and KUB chicken samples compared to the mice samples (P <0.05). The sperm motility values from three different species were 81.13 ± 1.64% for the PO bull samples, 83.88 ± 1.25% for the KUB chicken samples, and 66.13 ± 1.64% for the mice samples (Table 1). The sperm viability range between 63.75 ± 1.39% to 85.86 ± 1.96% (Table 1). The sperm viability values from three different species were 83.38 ± 1.69% for the PO bull samples, 85.86 ± 1.96% for the KUB chicken samples, and 63.75 ± 1.39% for the mice samples (Table 1). The percentage of viability in KUB rooster and PO bulls showed relatively similar values, and there is no significant difference (P >0.05). The viability rates of Peranakan Ongole breed bulls and KUB chickens were relatively higher than the mice, and there was a significant difference (P <0.05). The sperm membrane integrity values from three different species were 70.86 ± 1.13% for the PO bull samples, 87.25 ± 1.49% for the KUB chicken samples, and 59.00 ± 1.07% for the mice samples (Table 1). Percentage values of membrane integrity KUB Chicken and Peranakan Ongole breed bulls were higher than the mice (Table 1, Figure 1), and there was a significant difference between the KUB chickens and Peranakan Ongole breed bulls compared to the mice samples (P <0.05).
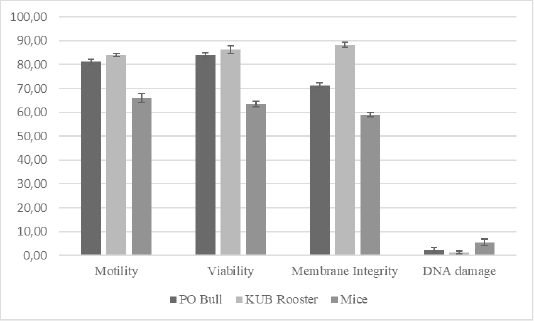
Figure 1: The total average of semen quality parameters from Peranakan Ongole breed bulls, KUB chickens, and mice. Error bar indicates standard deviation.
In three different samples (PO bull, KUB rooster, and mice), individual differences in DNA fragmentation results were found, we investigated the variance of the sperm DNA damage. These were the first study findings that showed different results in three different semen samples. The table showed the DNA fragmentation range values between 1.50 ± 0.53% to 5.50 ± 1.51%. The sperm DNA fragmentation values from three different species were 2.00 ± 0.93% for the PO bull samples, 1.50 ± 0.53% for the KUB chicken samples, and 5.50 ± 1.51% for the mice samples (Table 1). The mice sperm DNA fragmentation had the highest values, and there was a significant difference between the mice to others (P <0.05). The analysis found that the percentage of fresh semen motility, viability, and membrane integrity had positive correlations with one another. On the contrary, the DNA damage showed negative correlations with others (Table 2).
Overall, KUB chickens had the lowest rates of sperm DNA damage. However, the DNA damage has probably stayed inherent to each semen. The sperm DNA damage rate in mice was the highest at a range between 5-8% among the Peranakan Ongole breed bulls and KUB chickens ranging from 1-3%. Numerous observations might affect the sample findings. This study also found that the TUNEL assay was not qualified to assess the semen of KUB chickens because the results from the fluorescent microscope were not as clear as the other samples (Figure 2). Other evaluation methods such as comet assay may the better methods to evaluate the sperm DNA fragmentation from chicken samples, as previously researched by Gliozzia et al. (2011). The mice semen samples showed the clearest fluorescent microscope results among others (Figure 2), though the main factor remains unknown. Which findings proved that different species samples and semen collecting methods might affect sperm DNA damage. Besides, the sperm DNA damage was not correlated with the semen quality parameters as sperm motility, viability, and membrane integrity. This finding is also in line with Takeda et al. (2015) which states that the results from the sperm TUNEL index were not correlated to conventional semen quality parameters. In several studies including this research, the fresh semen was evaluated without washing procedures. Although this method caused dead spermatozoa to be included in the analysis, such a procedure will not separate the spermatozoa with higher motility, and more valid results can be obtained. Fresh semen that is evaluated with washing procedures or centrifugation may reveal different results.
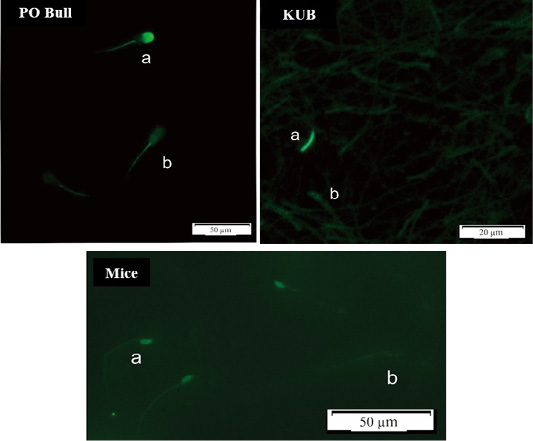
Figure 2: DNA Fragmentation results using the TUNEL assay method, spermatozoa in a bright green fluorescence showed damaged (fragmented) DNA (a), while spermatozoa in a dull green fluorescence showed normal DNA (b).
The DNA sperm cell is more susceptible to injury because of highly compact and stable chromatin compared to somatic cells with lower dense chromatin characteristics (Gliozzia et al., 2011). Chromatin condensation is an essential factor in the maturation process. It depends on the exchange between histones and protamines. The sperm ratio of histones and protamines depends on the variety and characteristics of species (Balhorn, 1982). This study found that KUB chicken had the lowest rates of sperm DNA damage. Some species lacked cysteine residues which cause high rates of DNA damage (Chiva et al., 1987). Though the used procedures were different, DNA damage did not correlate to other semen quality parameters (Table 2). Regardless of the sperm with lower motility and viability rate, it might have had high rates of DNA integrity. Previous studies in humans observed there was a connection between the comet assay, DNA strand breaks detection, and SCSA with the sperm chromatin resistance test to acid denaturation (Schlegel and Paduch, 2005; Aravindan et al., 1997). According to sperm cell studies, an increased denaturation sensitivity of sperm cells may lead to substantial DNA chain damage. It has partially been responsible for the denaturation susceptibility development of DNA sperm (Sailer et al., 1995).
A study found that infertile bulls had sperm DNA damage rates up to 25%, contrary to the fertile bulls with less than 15% of sperm DNA damage (Anzar et al., 2002). It might presume that sperm DNA damage < 20% will not influence artificial insemination. Another study proved the TUNEL assay was an efficient technique to determine the consistency of young Norwegian red bulls’ semen which concerns the pregnancy rate as a result of AI (Waterhouse et al., 2006). The present study result overview was in line with the human spermatozoa results (Zribi et al., 2010; Thomson et al., 2009). In humans, oxidative stress is more likely affecting sperm DNA fragmentation compared to others as caspase and apoptotic sequelae activation (Martin et al., 2004). Oxidative stress may affect every single part of sperm cells as lipids, proteins, nucleic acids, and sugars. Long-term lipid peroxidation can degrade the matrix structure of lipids. It causes the cell membranes to become unstable, disrupts the membrane function, and reduced membrane fluidity (Prihantoko et al., 2020b). The spermatozoa of bulls, with less than 4% of DNA damage, tend to have 10% higher performance of AI results as compared to the average rates (Takeda et al., 2015). In this study, evaluation of the three species sperm samples showed less than 10% of DNA damage. It is concluded that there are no correlations between sperm DNA damage, motility, and membrane integrity (%). Similar findings have been published previously (Trisini et al., 2004). As for comparisons, other relevant studies did not report similar correlations or discordant results (Morris et al., 2002). Moreover, other methods may cause oxidative stress that leads to sperm DNA damage and affect semen quality. Another factor remains unknown, but it still can cause effects while the conventional semen parameters may not always associate with the DNA status.
Table 1: Total average of fresh semen quality parameters and DNA damage from Peranakan Ongole breed bulls, KUB chickens, and mice.
| Samples | Motility (%) | Viability (%) | Membrane integrity (%) | DNA damage (%) |
|
PO bull |
81.13 ± 1.64a |
83.38 ± 1.69a |
70.86 ± 1.13b |
2.00 ± 0.93ab |
| KUB chicken |
83.88 ± 1.25a |
85.86 ± 1.96a |
87.25 ± 1.49a |
1.50 ± 0.53a |
| Mice |
66.13 ± 1.64c |
63.75 ± 1.39c |
59.00 ± 1.07c |
5.50 ± 1.51c |
a,b,c Different lowercase superscripts in the same row show the significant difference (P <0.05).
Table 2: The correlation rates among different parameters and DNA damage from Peranakan Ongole breed bulls, KUB chickens, and mice.
| Parameters |
Correlation coefficients |
||
| PO bull | KUB chicken | Mice | |
| Motility and Viability | 0.910** | 0.870** | 0.767* |
| Motility and Membrane integrity | 0.705* | 0.712* | 0.814* |
| Motility and DNA damage | -0.188 | 0.107 | 0.201 |
| Viability and Membrane integrity | 0.781* | 0.747* | 0.770* |
| Viability and DNA damage | -0.183 | 0.341 | -0.204 |
| Membrane integrity and DNA damage | -0.274 | -0.180 | 0.265 |
*= Significant at P<0.05; ** = Significant at P<0.01
CONCLUSIONS AND RECOMMENDATIONS
The TUNEL assay is reliable to assess DNA fragmentation in bulls and mice spermatozoa but not for the chickens. The sperm DNA fragmentation in mice showed the highest rates than the bulls samples. DNA damage or sperm DNA fragmentation assessment is an advanced parameter to determine sperm quality.
ACKNOWLEDGMENTS
This study is supported by Gadjah Mada University and the Ministry of Research, Technology and Higher Education, the Republic of Indonesia.
Novelty Statement
Application of the TUNEL assay method on semen samples from Peranakan ongole bulls and KUB chicken has never been done before. The first comparison between three different animal samples (bull, chicken, and mice) for sperm DNA fragmentation.
AUTHOR CONTRIBUTION
All authors participate from the beginning of this study until the publications are released.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES





