Advances in Animal and Veterinary Sciences
Research Article
Genetic Virulence Determinants and Antimicrobial Susceptibility Profile of Escherichia coli Isolated from Some Milk Products
Hala S.H. Salam1, Asmaa El-Sayed Zaghloul2, Esraa G. Hefny3, Essam I. Eltoukhy4, Abdelhafez Samir5, Abeer A.E. Shehata6*
1Department of Bacteriology, Mycology and Immunology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt; 2Directorate of Veterinary Medicine, El-Fayoum, Egypt. 3Food Hygiene Department, Animal Health Research Institute, ARC Research Institute, Egypt; 4Biotechnology Department, Animal Health Research Institute, ARC, Egypt; 5NLQP, Animal Health, ARC, Egypt; 6Department of Bacteriology, Animal Health Research Institute, El-Fayoum Laboratory, El-Fayoum, Agricultural Research Center, Egypt.
Abstract | Escherichia (E.) coli is a highly versatile bacterial species habitats intestinal tract of warm-blooded animals as a normal flora and it can cause severe illnesses in different animal species and human being. Dairy products are considered a source of E. coli to humans. In humans, it could cause variety of diseases ranges from bloody diarrhea to hemolytic uremic syndrome. This study aimed at studying the prevalence of E. coli in yoghurt, kariesh cheese and cream, inspecting the prevalent E. coli serogroups, investigating their antimicrobial susceptibility profile using the disk diffusion test and determining some of its virulence genes. A total of 155 samples were collected (50, 50 and 55 from yoghurt, cream and kariesh cheese, respectively) from local markets in El-Fayoum Governorate, Egypt. The prevalence of E. coli in yoghurt, cream and kariesh cheese were 12.0, 56.0 and 61.8%, respectively. There were 11 different serogroups of E. coli amongst the inspected isolates. Serogroups O: 55, O: 114 and O: 125 were identified in the whole examined products, while serogroups O: 26, O: 27 and O: 78 were identified in yoghurt and kariesh cheese only. Antimicrobial resistance against ampicillin, streptomycin, trimethoprim-sulfamethoxazole, cefotaxime, nalidixic acid, tetracycline, and amoxicillin-clavulanic acid were 11.8, 10.3, 8.8, 7.4, 5.9, 4.4, and 4.4%, respectively. Moreover, multidrug resistance was noted in 10.3% of the inspected E. coli isolates. PCR revealed the presence of astA, eaeA, stx1 and stx2 genes in 100, 50, 20 and 10%, respectively of the tested isolates. The present study clarify that yoghurt, kariesh cheese and cream to be potential sources of various E. coli pathotypes harboring virulence factors able to induce lethal diseases in humans. Moreover, multidrug resistant strains of E. coli that even if non-pathogenic will participate in establishing resistance in gastrointestinal tract bacterial community and subsequently environment. So, there is a fundamental need to follow the implementation of both good hygiene and manufacturing practices as well as application of strict hazards analysis and critical control point in dairy products industry for the sake of human safety.
Keywords | E. coli, Virulence, Genes, Serogroupe, Cheese
Received | May 21, 2021; Accepted | July 05, 2021; Published | November 01, 2021
*Correspondence | Abeer Ahmed El-Sayed Shehata, Department of Bacteriology, Animal Health Research Institute, El-Fayoum Laboratory, El-Fayoum, Agricultural Research Center, Egypt; Email: aae_shehata@yahoo.com
Citation | Salam HSH, Zaghloul AE-S, Hefny EG, Eltoukhy EI, Samir A, Shehata AAE (2021). Genetic virulence determinants and antimicrobial susceptibility profile of Escherichia coli isolated from some milk products. Adv. Anim. Vet. Sci. 9(12): 2139-2146.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2139.2146
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Salam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Escherichia coli (E. coli) is Gram-negative bacilli belongs to the family Enterobacteriaceae. E. coli strains habitat intestinal tract (Lara et al., 2016) of both animal and humans normally as a non-pathogenic bacilli. Although most of E. coli strains are non-pathogenic, some strains are well armed with a variety of virulence factors that are diverge in accordance to the pathotype of E. coli.
Pathogenic E. coli have been classified into two categories; the diarrheagenic E. coli (DEC) and the extraintestinal pathogenic E. coli (ExPEC). Among the diarrheagenic E. coli, there are currently six categories including enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), diffusively adherent E. coli (DAEC) and enterohemorrhagic E. coli (EHEC)/Shiga toxin-producing E. coli (STEC) (Xiaodong, 2010). While extra-intestinal pathogenic E. coli (ExPEC) can be classified into three categories, namely, uropathogenic E. coli (UPEC) causing urinary tract infection (UTI), meningitis-associated E. coli (MNEC) and necrotoxigenic E. coli (NTEC) which produces cytotoxic necrotizing factor (CNF) (Kaper et al., 2004).
Milk and milk products including (yoghurt, kariesh cheese and cream) are consumed worldwide. They classified as sources of great biological value protein, principal vitamins and minerals (Pereira, 2014). Consumption of raw milk and raw-milk products are widely distributed in several countries as well as Egypt (Ayad et al., 2004). On the other hand, they are considered as source of possibly injurious bacteria to humans, such as pathogenic E. coli (Oliver et al., 2005). E. coli can gain access to milk via fecal contamination or via direct secretion (mastitis) from udder into milk (Stephan and Kühn, 1999).
STEC represent a dangerous public health problem worldwide initiating several human gastrointestinal tract diseases, including watery or bloody diarrhea, and may lead to a life-threatening disease, such as haemorrhagic colitis (HC), thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) (Kalid and Andreoli, 2018). STEC strains yield two powerful cytotoxins initiating tissue damage in humans called Shiga toxins or verotoxins (stx1/vt1 and stx2/vt2) (Li et al., 2017). Stx2 producing strains are frequently linked to more severe infections (Muniesa et al., 2004).
Other virulence factor likewise, outer membrane protein intimin that is encoded by the eaeA gene and firmly attach and form attaching and effacing lesions to intestinal epithelial cells (Awad et al., 2020). Another dangerous aspect of pathogenic E. coli is the presence of the astA gene encoding enteroaggregative heat-stable enterotoxin 1 (EAST1) which was primarily distinguished in EAEC (Dubreuil, 2017). Afterward, astA gene was detected in another DEC pathotypes including EPEC, ETEC, and EHEC (Ménard and Dubreuil, 2002). The astA gene possibly is a significant virulence factor in DEC which could be injurious to humans (Hinenoya et al., 2014).
The present study aimed at investigating some dairy products (yoghurt, kariesh cheese and cream) manufactured and retailed under market situations for the prevalence of E. coli, prevalent serogroups, presence of some virulence genes. Additionally, to study their antimicrobial susceptibility profile against antimicrobial agents of veterinary and humans medicine concern.
MATERIALS AND METHODS
Samples
A total of 155 samples from different milk byproducts (50 yoghurt, 55 kariesh cheese and 50 cream samples) were collected from different supermarkets, retail and dairy shops in Al-Fayoum governorate, Egypt during the period from August 2019 to February 2020. Samples were transferred in sterile containers to the laboratory and analyzed directly on arrival for the isolation of E. coli.
Isolation and identification of E. coli
Isolation and biochemical identification of E. coli was done according to Collee et al. (1996).
Detection of hemolytic activity of the identified E. coli isolates
The ability of E. coli isolates to produce different types of hemolysin was phenotypically investigated using sheep blood agar 7% (Collee et al., 1996).
Serogrouping of the isolated E. coli
Serogrouping of E. coli isolates recovered from different milk byproducts was performed in accordance to Ewing (1986). I was performed in Department of Serology, Animal Health Research Institute, Agricultural Research Center, Egypt.
In vitro antimicrobial susceptibility testing of the identified E. coli strains
The isolated E. coli strains were investigated for their antimicrobial susceptibility profile using disk diffusion test against different antimicrobial classes of veterinary and human being significance. Second generation cephalosporin (cefoxitin, 30µg); third generation cephalosporin (cefotaxime, 30 µg); penicillin-inhibitor combination (amoxicillin-clavulanic acid, 30 µg); aminoglycosides (gentamicin, 10 µg and streptomycin, 10 µg); tetracyclines (tetracycline 10 µg); quinolone (nalidixic acid 30 µg); fluoroquinolone (ciprofloxacin, 5 µg); carbapenem (imipenem, 10 µg); and folate pathway antagonist (trimethoprim-sulfamethoxazole, 25 µg). All antimicrobial disks were obtained from Oxoid, UK. The in vitro antimicrobial susceptibility profiling and results interpretation were performed according to CLSI (2019).
Table 1: Oligonucleotide primer sequences of target genes specific for E. coli.
| Gene | Primer sequence (5'-3') | Amplicon size | Reference |
| stx1 | ACACTGGATGATCTCAGTGG | 614 bp | |
| CTGAATCCCCCTCCATTATG | |||
| stx2 | CCATGACAACGGACAGCAGTT | 779 bp | |
| CCTGTCAACTGAGCAGCACTTTG | |||
| eaeA | ATGCTTAGTGCTGGTTTAGG | 248 bp | |
| GCCTTCATCATTTCGCTTTC | |||
| astA | CCATCAACACAGTATATCCGA | 110 bp | |
| GGTCGCGAGTGACGGCTTTGT |
Table 2: PCR cycling conditions of the different primer sets.
| Gene | Primary denaturation | Secondary denaturation | Annealing | Extension | No. of cycles | Final extension |
| stx1 and stx2 | 94˚C 5 min. | 94˚C 30 sec. | 58˚C 40 sec. | 72˚C 45 sec. | 35 | 72˚C 10 min. |
| eaeA | 94˚C 5 min. | 94˚C 30 sec. | 51˚C 30 sec. | 72˚C 30 sec. | 35 | 72˚C 7 min. |
| astA | 94˚C 5 min. | 94˚C 30 sec. | 55˚C 30 sec. | 72˚C 30 sec. | 35 | 72˚C 7 min. |
Detection of some virulence genes in the prevalent E. coli serogroups isolated from dairy products using polymerase chain reaction (PCR)
Presence of astA, eaeA, stx1, and stx2 in the most prevalent serogroups of the isolated E. coli was done using PCR .
Preparation of DNA template
DNA template was obtained from overnight pure culture using QIAamp DNA Mini Kit (Catalogue no.51304) from Qiagen.
Amplification procedure
PCR reactions were performed in volumes of 25µL. Primers were obtained from Metabion (Germany) and master mix from Takara (Catalogue no. RR310). Table 1 reveals the used primer pairs for each gene, amplicon size and references used and Table 2 shows the cycling condition for each primer pairs. Ten microliters of the reaction products were analyzed by electrophoresis on 1% agarose gel containing ethidium bromide and results were visualized in a gel documentation system.
Statistical analysis
ANOVA test was used to investigate the prevalence of E. coli in different dairy products. Statistical significance was considered if p ≤ 0.05. All statistical comparisons were performed using IBM SPSS® Statistics software version 22.
RESULTS AND DISCUSSION
Prevalence of E. coli in different dairy products
E. coli prevalence (Table 3) differed according to the niche of dairy products. The highest prevalence (61.8%) was noted in kariesh cheese followed cream (56%), while the least prevalence was reported amongst yoghurt samples (12%). The overall prevalence of E. coli in the examined samples of the dairy products was 43.9%.
Table 3: Prevalence of E. coli isolated from dairy products.
| Dairy product | Samples No. | E. coli | |
| No. | %* | ||
| Yoghurt | 50 | 6 |
12.0§ |
| Kariesh cheese | 55 | 34 | 61.8 |
| Cream | 50 | 28 | 56.0 |
| Total number | 155 | 68 | 43.9 |
*%: Percentage was calculated according to the corresponding number of examined samples; §: Prevalence of E. coli in yoghurt was statistically lower than those reported in kariesh cheese and cream (p<0.05).
Prevalence of E. coli in yoghurt was statistically lower than those reported in kariesh cheese and cream (p<0.05). In contrast, kariesh cheese and cream E. coli prevalence difference was not statistically different (p>0.05).
Hemolytic activity of E. coli isolates
Alpha hemolysis was the only type of hemolysis phenotypically detected in E. coli isolated in the present study. Alpha-hemolytic activity was reported in 16 (23.5%) out of the inspected 68 isolates (one isolate recovered from cream and 15 isolates from kariesh cheese), while the remaining isolates were non-hemolytic i.e. gamma-hemolytic.
Serogrouping of E. coli isolates
Table 4 reveals the presence of 11 serogroups of E. coli amongst investigated 20 isolates that were selected to represent the examined dairy products (yoghurt, 5; kariesh cheese, 7 and cream, 8) under study (Table 4). Serogroups O55, O114 and 125 were reported in the investigated three dairy products.
Table 4: Serogrouping of E. coli isolates recovered from dairy products.
| Product | No. of isolates | Serogroup |
| Yoghurt | 5 | O25 |
| O55 | ||
| O114 | ||
| O125 | ||
| O128 | ||
| Kariesh cheese | 7 | O26 |
| O27 | ||
| O55 | ||
| O78 | ||
| O114 | ||
| O124 | ||
| O125 | ||
| Cream | 8 | O26 |
| O27 | ||
| O55 | ||
| O78 | ||
| O86 | ||
| O114 | ||
| O125 | ||
| O148 |
Antimicrobial susceptibility testing of E. coli recovered from dairy products
The in vitro antimicrobial susceptibility testing revealed diverse susceptibility/resistance behavior of the investigated E. coli against the tested antimicrobial agents (Table 5). All 68 tested isolates were 100% sensitive to gentamicin, imipenem, and cefoxitin. On the other hand, the resistance rates against ampicillin, streptomycin, trimethoprim-sulfamethoxazole, cefotaxime, nalidixic acid, tetracycline and amoxicillin-clavulanic acid were 11.8, 10.3, 8.8, 7.4, 5.9, 4.4 and 4.4%, respectively. Additionally, multidrug resistance was noted in seven out of 68 (10.3%) of the inspected E. coli isolates.
Detection of some virulence genes in the prevalence E. coli serogroups isolated from dairy products
The presence of astA gene was confirmed in the isolates (100%) under test (Figure 1), while eaeA was only defined in five out of ten (50%) investigated isolates (Figure 2). Regarding to shiga toxins, stx1 and stx2 were only defined in one (10%) and two (20%) of the tested isolates, respectively (Figure 3). Results clarify all isolates harboring shiga toxin genes also harbored astA and eaeA genes.
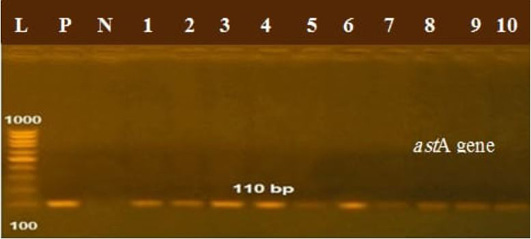
Figure 1: Lanes 1:10 positive amplification of astA gene at 110 bp P: positive control; N: negative control; L: DNA ladder.
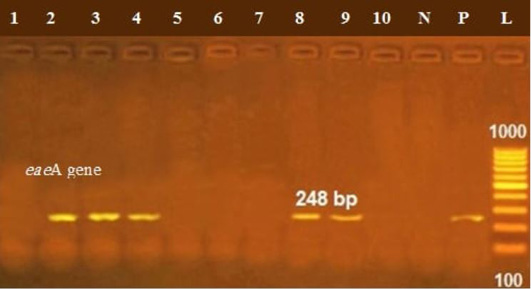
Figure 2: Lanes 2, 3, 7, 8, 9 positive amplification of eaeA gene at 248 bp; P: positive control; N: negative control; L: DNA ladder.
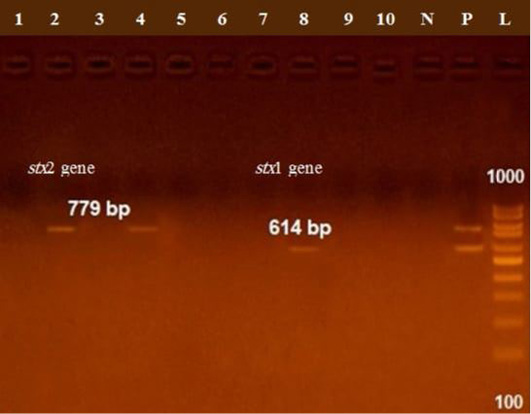
Figure 3: Lane 8 positive amplification of stx1 gene at 614 bp Lanes 2, 4 positive amplification of stx2 gene at 779 bp; P: positive control; N: negative control; L: DNA ladder.
Dairy products like yoghurt, cheese, and cream are widely consumed and markets have existed for them in many parts of the world for many generations. Dairy products over and above they are of high nutritional value for humans, they also offer an apt niche for bacterial growth likewise E. coli.
Table 5: Results of antimicrobial sensitivity test to 68 E. coli isolates from dairy products using the disk diffusion method.
| Class | Antimicrobial agent | Sensitive | Intermediate | Resistant | |||
| No. | % | No. | % | No. | % | ||
| Penicillin | Ampicillin | 60 | 88.2 | 0 | 0 | 8 | 11.8 |
|
ß-lactam/β-lactamase inhibitor combinations |
Amoxicillin-clavulanic acid | 65 | 95.6 | 0 | 0 | 3 | 4.4 |
| Third generation cephalosporins | Cefotaxime | 63 | 92.6 | 0 | 0 | 5 | 7.4 |
| Second generation cephalosporins | Cefoxitin | 68 | 100 | 0 | 0 | 0 | 0 |
| Carbapenems | Imipenem | 68 | 100 | 0 | 0 | 0 | 0 |
| Aminoglycosides | Gentamicin | 68 | 100 | 0 | 0 | 0 | 0 |
| Streptomycin | 54 | 79.4 | 7 | 10.3 | 7 | 10.3 | |
| Tetracyclines | Tetracycline | 63 | 92.6 | 2 | 2.9 | 3 | 4.4 |
| Quinolones | Ciprofloxacin | 66 | 97.1 | 2 | 2.9 | 0 | 0 |
| Nalidixic acid | 64 | 94.1 | 0 | 0 | 4 | 5.9 | |
| Folate pathway antagonist |
Trimethoprim-sulfamethoxazole |
61 | 89.7 | 1 | 1.5 | 6 | 8.8 |
%: Percentages were calculated in relation to the total number of tested isolates.
E. coli finds its way to milk and dairy products either, endogenously from the udder of diseased animal and or exogenously via direct contact with infected herds, environment or personnel (Farzana, 2009). In another consequence, E. coli is one of the main indicator organisms used for evaluating the quality of food (Anderson et al., 2006).
The present study revealed a diversity in the prevalence of E. coli in the examined dairy products (yoghurt, kariesh cheese and cream) (Table 3). The lowest prevalence was for yoghurt, 12% (6 isolates out of 50 samples) and it was significantly lower than those reported for kariesh cheese and cream (p<0.05). Low isolation rate of E. coli from yoghurt could be explained on the bases of the organic acid and low molecular weight antimicrobial substance produced by fermenting bacteria in yoghurt such as Lactobacillus spp. that showed in vitro antimicrobial activity against E. coli (Prabhurajeshwar and Chandrakanth, 2019).
Comparing the results of E. coli prevalence in yoghurt in the present study with other scholars’ result, identical prevalence of E. coli (12%) was noted by Okpalugo et al. (2008) in Abuja, Nigeria. On the other hand, Chaleshtori et al. (2017) reported closely matching isolation rate of E. coli (10%) from yoghurt in Iran. Higher isolation rates of E. coli form yoghurt were also reported 29.5, 44.8 and 88.0 % in Osun, Nigeria; El-Behera, Egypt and Mansoura city, Dakahlia Governorate, Egypt, respectively (El-Ansary, 2014; Abike et al., 2015; Kandil et al., 2018). Higher rates of E. coli from yoghurt in different studies could be attributed to the initial load of the milk before processing; usage of mastitic milk (Awadallah et al., 2016), improper sanitation of equipment used during processing; contamination after processing by unhygienic handling, packaging material (Pal et al., 2018) or to the storage temperature and time elapsed from manufacturer till sampling (Bachrouri et al., 2006).
On the other hand, the prevalences of E. coli in kariesh cheese and cream were 61.8 and 56%, respectively. Higher isolation rates of E. coli from kariesh cheese and cream could be attributed to the preparation of these products from mastitic milk (Awadallah et al., 2016) raw milk with high bacterial count, probiotic bacteria are not used in their preparation and also external contamination could occur at one or more points during processing (Deschenes et al., 1996). Non-statistical significant (p>0.05) of E. coli isolation rates from kariesh cheese and cream could be attributed to preparation of cream and kariesh cheese from the same milk source. Additionally, previous studies reported a range of 40-75% and 37.5-76.7% isolation rates of E. coli from kariesh cheese and cream in different regions in Egypt (Abd El-Tawab et al., 2020; Baraheem et al., 2007; El Nahas et al., 2015; Ibrahim et al., 2019).
Out of 20 E. coli isolates represented the dairy products under study 11 serogroups (Table 4) were identified (O25, O26, O27, O55, O78, O86, O114, O124, O125, O128, O148). El-Bagory et al. (2004) identified verotoxigenic E. coli O26 from the examined yoghurt samples. Also, Abike et al. (2015) found O26, O55, O86, O114, and O128 serogroups in raw milk, yoghurt and cheese. In a previous study, similar E. coli serogroups (O26, O55, and O114) were recovered from kariesh cheese and (O26, O55 and O114) form cream (El Nahas et al., 2015). Many scholars (Scott et al., 2009; Osman et al., 2013; Shehata and Salam, 2012; Awadallah et al., 2016) reported different E. coli serogroups either in diarrheic calves, healthy cattle or mastitic milk (O25, O26, O55, O78, 86, O114, O125, O148).
All isolates of E. coli recovered from dairy products in the present study were tested for their susceptibility behavior against 11 antimicrobial agents represented different antimicrobial classes of human being and veterinary concern in the region under study. The in vitro antimicrobial susceptibility testing revealed diverse susceptibility/resistance behavior of the investigated E. coli isolates against the tested antimicrobial agents (Table 5). All 68 tested isolates were 100% sensitive to gentamicin, imipenem, and cefoxitin. On the other hand, the resistance rates against ampicillin, streptomycin, trimethoprim-sulfamethoxazole, cefotaxime, nalidixic acid, tetracycline, and amoxicillin-clavulanic in descending order were 11.8, 10.3, 8.8, 7.4, 5.9, 4.4 and 4.4%, respectively. Side by side, growing of resistance was observed by the intermediate behavior of the investigated isolates against the tested antimicrobial agents. The percentages of the intermediate zones in ascending order were 1.5, 2.9 and 10.3% against trimethoprim-sulfamethoxazole, ciprofloxacin and streptomycin in turn. Additionally, multidrug resistance was noted in seven out of 68 (10.3%) of the inspected E. coli isolates.
Results divulged the correlation between the used antimicrobial agents in veterinary medicine and the reporting of resistance in isolates of veterinary origin and vice versa. Cefoxitin, imipenem and ciprofloxacin are of notorious use in medication of large animals and this could explain the results of 0.0% resistance records against these antimicrobial agents. Although the development of intermediate sensitivity behavior against ciprofloxacin and this could be due to the use of ciprofloxacin in broiler industry (Jónsdóttir and Kristinsson, 2008) that can later finds its way to the environmental niche either to humans or other animals. On the other hand, some antimicrobial classes showed higher rates of either resistance or intermediate behavior against the tested E. coli isolates likewise, penicillins, aminoglycosides, and tetracyclines, quinolones (first generation) and sulfonamides as these agents are of wide use in veterinary sectors and listed by the OIE as antimicrobial agents of veterinary importance (OIE, 2019). Furthermore, this could explain the prevalence of multidrug resistance amongst the tested isolates (10.3%).
Higher prevalence rates of resistance were reported in Mansoura city, Egypt against streptomycin, nalidixic, cefotaxime, tetracycline, trimethoprim-sulfamethoxazole, ampicillin, ciprofloxacin and gentamicin 100, 80, 60, 60, 60, 40, 40 and 20% in descending order (El-Baz, 2019). Also, Abd El-Tawab et al. (2020) reported 16.7% resistance amongst the E. coli tested against tetracycline in El-Gharbia Governorate, Egypt. There is a direct correlation between the abuse of antimicrobials and emergence of resistance amongst bacterial communities (Aly, 2013) and this could expound the metamorphosis in resistance profile of E. coli in different areas.
E. coli tempt its pathogenic actions through versatile sets of virulence elements that work in harmony to produce various illnesses in animals and humans. Phenotypic detection of hemolysis revealed the presence of α-hemolysin in 23.5% of the inspected isolates that is an exotoxin produced by E. coli and enhances virulence in clinical infections (May et al., 2000). Side by side, genotypic investigation revealed the presence of astA, eaeA, stx1 and stx2 genes in variable rates in ten E. coli isolates represented those isolated from dairy products under investigation (Figures 1, 2 and 3). All isolated had astA gene, eaeA was represented in five isolates while stx1 and stx2 were only noticed in two and one isolates, respectively. EAST1 induced by astA gene associates diarrheagenic E. coli in humans and animals and other scholars noted there presence even with non-diarrheagenic E. coli (Hinenoya et al., 2014) isolated from healthy cattle and swine. All shiga toxin genes (either stx1 or stx2) positive isolates were positive also for eaeA gene. This makes dairy products act as a potential source of STEC for humans as eaeA genes induce adhesion protein secretion required for intimate adherence of E. coli (Blank et al., 2002) that give chance for the cells of E. coli to produce shiga toxins to induce either watery or bloody diarrhea and may lead to a lethal disease such as HC, TTP and HUS (Khalid and Anderoli, 2018).
Researchers in different regions reported astA, eaeA, stx1 and stx2 genes in E. coli isolated from dairy products with variable prevalence rates (Elafify et al., 2020; Dehkordi et al., 2014) which could be attributed to the difference in the circulating serotypes in every study area.
CONCLUSIONS AND RECOMMENDATIONS
The present study points to yoghurt, kariesh cheese and cream as serious potential source of various E. coli pathotypes harboring virulence factors able to induce lethal diseases in humans. Moreover, multidrug resistant strains of E. coli that even if non-pathogenic will participate in establishing resistance in gastrointestinal tract bacterial community and environment. So, there is a fundamental need to follow the implementation of both good hygiene and manufacturing practices as well as application of strict hazards analysis and critical control point in dairy products industry for the sake of human safety.
Author’s Contribution
All authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES





