Advances in Animal and Veterinary Sciences
Research Article
Combined Controlled-Internal Drug Release and Ovsynch Synchronization Protocol Does not Improve Fertility Outcome In Buffaloes
Ahmed Saad A. Hassaneen1*, Ayman M. Ibrahim2, Nasra Ahmed M. Yousef1, Ahmed Ezzat Ahmed1, 3*
1Department of Theriogenology, Obstetrics and Artificial Insemination, Faculty of Veterinary Medicine, South Valley University, Qena 83523, Egypt; 2Veterinarian, Luxor Governorate, Egypt; 3Biology Department, Faculty of Science, King Khalid University, 61413 Abha, Saudi Arabia.
Abstract | This study aimed to determine whether combination of the controlled-internal drug releasing device (CIDR) within or before ovsynch protocols in acyclic postpartum buffaloes improves both the resumption of cyclicity and conception rate compared to ovsynch protocols only. Thirty-one clinically healthy female buffaloes (3-5 years-old; and 440.0± 10.0 kg b.wt) suffering from prolonged postpartum anestrous (3-9 months) were included. Buffaloes with any disorders that may directly or indirectly affect reproductive performance were excluded. Blood sampling was performed at these time-points; day -10, 0, 4, 7, and 9. Plasma progesterone and phosphorus (P) were assayed. Our results showed that the conception rate was significantly higher (P<0.05) in the GPG ovsynch-treated compared to the G-CIDR-PG but not the CIDR-GPG modified ovsynch-treated modified with conception rates of 57.1%, 30.0%, and 50.0 %respectively). This study found that, the P concentrations were significantly higher in the responsive compared to the non-responsive CIDR-GPG-modified ovsynch-treated group. This study concluded that GPG-ovsynch would be beneficial for synchronization in postpartum buffaloes. Moreover, CIDR-GPG likely improve the fertility, since the higher P levels can be utilized in activating the follicular dynamics.
Keywords | Anoestrus, Fertility, Gonadotropins, Progesterone-induced estrus.
Received | August 12, 2021; Accepted | August 18, 2021; Published | October 01, 2021
*Correspondence | Ahmed Saad A Hassaneen, Department of Theriogenology, Obstetrics and Artificial Insemination, Faculty of Veterinary Medicine, South Valley University, Qena 83523, Egypt; Email: aabdelrahman@kku.edu.sa, ahmed.ezzat@vet.svu.edu.eg
Citation | Hassaneen ASA, Ibrahim AM, Yousef NAM, Ahmed AE (2021). Combined controlled-internal drug release and ovsynch synchronization protocol does not improve fertility outcome in buffaloes. Adv. Anim. Vet. Sci. 9(12): 2027-2035.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2027.2035
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Ahmed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Buffaloes have an important role in agricultural economy in several Asian and African tropical and subtropical countries through providing both milk and meat, in addition to, their draught power. Buffaloes share about half of the overall dairy production in Egypt (Borghese, 2005; Perera, 2011). Buffaloes’ milk is preferable in Egypt because of its high fat and solid non-fat contents which gives the milk a good taste compared to that is of cows. Moreover, rearing of buffaloes is favored by because of their better adaptation to tropical and subtropical environment (Abdalla, 2003; El-Wishy, 2007).
Buffaloes’ production is largely affected by reproductive performance. Low reproductive efficiency of Egyptian buffaloes is mainly due to poor estrus detection and prolonged inter-calving interval (Singh et al., 2000; El-Wishy, 2007). Longer inter-calving intervals is mainly due to prolonged postpartum anestrus which is mainly attributed to ovarian inactivity (Hattab and Osman, 2000; Shah and Nakao, 2010; Khan et al., 2012). It should be noted that the adaptation of the buffaloes on the harsh environment results in adverse effects on the reproductive efficiency resulting in prolonged postpartum anoestrus, low conception, and long inter-calving intervals (Perera, 2011).
Although buffaloes are polyestrous, their reproductive activity has wide variation throughout the year with a distinct seasonal difference in expressing estrus, and conception rate (Zicarelli, 1997). Furthermore, estrus in buffaloes is more frequent and stronger during short day seasons (Barkawi et al., 1989). Reduced reproductive activity of buffaloes during summer months has been associated to some factors such as day length (Dessoukey and Juma, 1990), and heat stress (Warriach et al., 2008). Endocrinologically, summer anestrus in buffaloes is characterized by low plasma concentrations of both the pituitary and ovarian hormones (Jainudeen and Hafez, 1993; Kaur and Arora, 1994). Economically, prolonged postpartum anestrous and delayed resumption of ovarian activity lead to the major economic losses to the buffalo breeders (Singh et al., 2000; El-Wishy, 2007; Stanimir et al., 2012). It should be noted that one third of postpartum buffaloes remain anestrous for more than 5 months (El-Wishy, 2007).
Estrous synchronization depends on either lengthening or shortening the estrous cycle. Different techniques based on several hormonal treatments including P4, PGF2α and gonadotropins are used (Ryan et al., 1995). For successful synchronization, a clear understanding of the hormonal regulation of the estrous cycle is required. In buffaloes, the hormonal profile is generally like that in cows with lower peak concentrations of P4 and E2 plasma levels (Baruselli, 2001; de Rensis and López-Gatius, 2007). The success rate of synchronization in buffaloes is poor during anoestrus (Metwelly, 2006). Most of the recent synchronization programs for buffaloes are based on similar regimes applied in cows, however, the main difference is that buffaloes experience a more intense low breeding season during the summer, when cyclic ovarian activity is reduced (Neglia et al., 2003; Presicce et al., 2004; Campanile et al., 2007). Different synchronization protocols with or without timed artificial insemination (TAI) have been utilized for resuming the ovarian activity, however, the obtained outcomes were conflicting (Zaabel et al., 2009; Karen and Darwish, 2010; Azawi et al., 2012a, b, c; Waqas et al., 2016; Sharma et al., 2017).
Different synchronization programs (Nayak et al., 2009; Naseer et al., 2012), breed and age of buffaloes (Singh et al., 2000; Derar et al., 2012) seasonal or nutritional effects (Nanda et al., 2003) are possible reasons for such differences in the outcomes.
The efficacy of the PGF2α-based regimens treatment in buffaloes depends upon plasma P4 concentration, the CL size, and the follicular status before PGF2α administration (Brito et al., 2002). In buffaloes, the effect of PGF2α administration is very similar to that observed in cattle. Pregnancy rate following PGF2α-induced estrus is about 50% during high breeding season (de Araujo, 2002; Neglia et al., 2003) like that obtained after natural estrus (Sahasrabudhe and Pandit, 1997). The efficacy of PGF2α treatment is dramatically reduced during the low-breeding season, with conception rates below 25% (Chohan et al., 1995), even when majority of treated buffaloes (88%) display standing estrus (Sahasrabudhe and Pandit, 1997).
Ovsynch and GnRH-based regimens in buffaloes induce ovulation in 60–90% of treated animals (Aboul-Ela et al., 1985; Barkawi et al., 1993; Baruselli, 2001; Paul and Prakash, 2005) with conception rates during the high-breeding season between 33% (Paul and Prakash, 2005) and 60% (Baruselli, 2001). The degree of synchronization success of ovsynch in cyclic animals requires the presence of a dominant follicle at the time of the first GnRH treatment (Thatcher et al., 2001; de Rensis et al., 2005).
The use of P4-based regimens (CIDR) include the administration of GnRH or E2 at the time of CIDR insertion and/or PGF2α at the day of removal. Estrus has been observed in 80–93% of treated buffaloes after about 2-5 days (Bartolomeu et al., 2004; Barile et al., 2001), with pregnancy rate varies from 20 (Baruselli, 2001) to 50% (Barile et al., 2001; Neglia et al., 2003) during high-breeding season.
P4-releasing intravaginal device (PRID) and CIDR have been used with ovsynch programs for treatment of buffaloes suffering from inactive ovaries (Yendraliza Zesfin et al., 2011; Barile, 2012). After device removal, the plasma P4 concentration sharply decrease and those the GnRH, LH and FSH get increase leading to resumption of the ovarian activity (Zerbe, 1999). These regimes have been applied for both cyclic and a cyclic buffalo cows (Ali and Fahmy, 2007; Karen and Darwish, 2010) however, the conception and pregnancy rate are still unsatisfactory.
The ovarian activity of buffalo is influenced by deficiency of some minerals like phosphorus (P). Deficiency of P is associated with infertility and anoestrus conditions in cows (Hidiroglou, 1979). Heat stress could result in higher cortisol which is known to decrease serum P (Koch et al., 1961). Higher P values in normal cyclic animals had been previously recorded (Newar et al.,1999).
The overall question of our study was whether combination of the CIDR with ovsynch protocols in acyclic buffalo improves both the resumption of cyclicity and conception rate compared to ovsynch protocols only. The current study aimed to evaluate the effect of P4 supplementation in ovsynch protocols in postpartum anestrous buffalo and to evaluate the efficacy of three different synchronization protocols; GPG ovsynch, G-CIDR-PG modified-ovsynch, and CIDR-GPG modified-ovsynch in anestrus buffaloes with evaluation of subsequent conception rate.
MATERIALS AND METHODS
Study location
This study was conducted in Luxor governorate, Egypt, located at 76 m above mean sea level, latitude 25.69° N and longitude 32.64° E. The study was performed during the low-breeding season (May–October) when the low (minimum) and high (maximum, °C) temperature ranged between 19-24°C and 35-41°C, respectively.
Animals
Thirty-one clinically healthy female buffaloes (3-5 years-old; and 440.0± 10.0 kg b.wt) suffering from postpartum anestrous (3-9 months) were included in this study. The anestrus had been confirmed by repeated rectal examination. Female buffaloes with history of metritis, lameness, or any other disorders that may affect the reproductive performance were excluded.
All female buffaloes were milked twice daily at 07:00 am and 07:00 pm with average low milk yield of 4 kg/head.
Experimental design
All female buffaloes were randomly subjected to one of the three different synchronization programs as follow:
GPG-ovsynch-treated group (n = 7):
Female buffaloes (n = 7; 3-5 years-old; and 391.71 ± 18.33 kg b.wt) were subjected to GnRH-PGF2α-GnRH ovsynch protocol. Animals were subjected to intramuscular injection of 0.02 mg buserelin acetate, a natural GnRH analogue (Receptal®, Intervet international, European union) on day 0. Seven-days later, animals were intramuscularly treated with 25 mg dinoprost tromethamine (Lutalyse, Pfizer Animal Health, Pfizer Manufacturing, Belgium), and finally 0.02 mg buserelin acetate was administered on day 9 followed by TAI (Figure 1a).
G-CIDR-PG-modified ovsynch-treated group (n = 10):
Female buffaloes (n = 10; 3-5 years-old; and 461.50 ± 12.10 kg b.wt) were subjected to GnRH-CIDR-PGF2α-GnRH modified ovsynch protocol. buffaloes were subjected to intramuscular injection of 0.02 mg buserelin acetate, on day 0, followed by insertion of controlled-internal drug releasing device (CIDR containing 1.38 gm of P4 (EAZI-BREED CIDR® cattle insert, Pharmacia & Upjohn Company Kalamazoo, Michigan, USA) Registered to and marketed by: Pfizer New Zealand Ltd) for 7 days. On day 7, animals were intramuscularly treated with 25 mg dinoprost tromethamine, and finally 0.02 mg buserelin acetate was administered on day 9 followed by TAI (Figure 1b).
CIDR-GPG-modified ovsynch-treated group (n = 14):
In this 3rd group, female buffaloes (n = 14; 3-5 years-old; 445.90 ± 15.50 kg b.wt) were subjected to CIDR-GnRH-PGF2α-GnRH modified ovsynch protocol. Animals were subjected intravaginal CIDR for 7 days which is prior to intramuscular injection of 0.02 mg buserelin acetate, on day 0. On day 7, animals were intramuscularly treated with 25 mg dinoprost tromethamine, and finally 0.02 mg buserelin acetate was administered on day 9 followed by TAI (Figure 1c).

Figure 1: Ovsynch and modified ovsynch synchronization protocols show the GnRH-PGF2α-GnRH (GPG) ovsynch program with GnRH administration only at day 0 (A), GnRH-CIDR-PGF2α-GnRH (G-CIDR-PG) synchronization program with GnRH at day 0, CIDR insertion for 7 days from day 0 to 7 (B), and CIDR-GPG synchronization program with CIDR insertion for 7days from day -7 to 0, GnRH at day 0 (C), followed by PGF2α at day 7, GnRH at day 9, and timed artificial insemination after 16-18 hours.
Timed artificial insemination (TAI)
Frozen buffalo-bull semen straws (El-Abasia AI lab. El-Dokki, Cairo, Lot number: 565, progressive forward motility of at least 45%) were used for insemination.
Blood sampling
Five-ml blood samples were collected from the jugular vein into vacutainer heparinized tubes at these time-points; day -10, 0, 4, 7, and 9. Blood samples were centrifuged at 3000 rpm for 15 min. After being separated, plasma samples were stored at - 20ºC until further assay.
Progesterone (P4) assay
Plasma P4 concentrations were measured by single enzyme-linked immune sorbent assay (ELISA) using P4 kits (ELITechGroup: In Vitro Diagnostic Equipment & Reagents, France).
Phosphorus (P) assay
Plasma P concentrations were measured by using P kits (spectrum–Egyptian company for biotechnology, Egypt) by means of the spectrophotometer machine (Yıldız and Öcal, 2001).
Pregnancy diagnosis
For evaluation of the post-synchronization fertility parameter, pregnancy diagnosis was performed about 45-60 days post-AI.
Statistical analysis
All data were presented as the mean ± S.E.M. The animals within each group were classified as responsive and non-responsive subgroups based on the P4 concentrations where plasma P4 levels >1.0 ng/ml at day 7 were considered as responsive. The statistical significance of differences in plasma P4 and P concentrations between the subgroups throughout the days of study were determined by student t-test. All data were analyzed by using Graph-Pad Prism (GraphPad Software, San Diego, CA, USA). Results were considered significant at the P<0.05 level.
RESULTS
In the present study, three different ovsynch and modified ovsynch protocols were used to synchronize estrous in postpartum anestrous buffalo. Our results showed that the conception rate was significantly higher (P<0.05) in the GPG ovsynch-treated compared to the G-CIDR-PG but not the CIDR-GPG modified ovsynch-treated modified with conception rates of 57.1%, 30.0%, and 50.0 %respectively).
Plasma P4 and P concentrations in GPG-ovsynch-treated group: Plasma P4 concentrations were significantly higher (P<0.05) at day -10, 4, and 7 in responsive group in comparison to those in non-responsive group (Figure 2a). While, no difference was reported in the plasma P concentrations between both sub-groups (Figure 2b).

Figure 2: Plasma progesterone (P4) and plasma phosphorus (P) concentrations in GPG-ovsynch-treated group. (a) Plasma P4 concentrations for both responsive and non-responsive groups. Zero (0) means day of first dose GnRH administration, and black arrow indicates day of PGF2α injection. (b) Plasma P concentrations for both responsive and non-responsive groups throughout the period of study. Asterisk means a significant difference at P<0.05. N.S. means non-significant difference. All values are presented as mean ± SEM.
Plasma P4 and P concentrations in G-CIDR-PG-modified ovsynch-treated group: Plasma P4 concentrations were significantly higher (P<0.05) at day 0, 4, and 7 in responsive group in comparison to those in non-responsive group (Figure 3a). While, there was a non-significant difference in plasma P concentrations between responsive and non-responsive groups (Figure 3b).
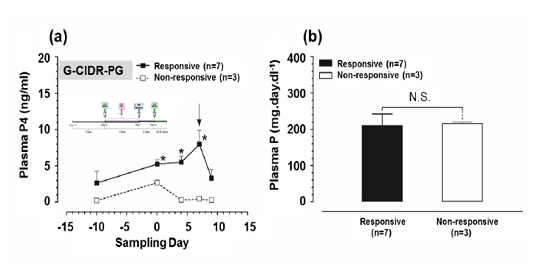
Figure 3: Plasma progesterone (P4) and plasma phosphorus (P) concentrations in G-CIDR-PG-modified ovsynch-treated group. (a) Plasma P4 concentrations for both responsive and non-responsive groups. Zero (0) means day of first dose GnRH administration, and black arrow indicates day of PGF2α injection. (b) Plasma P concentrations for both responsive and non-responsive groups throughout the period of study. Asterisk means a significant difference at P<0.05. N.S. means non-significant difference. All values are presented as mean ± SEM.
Plasma P4 and P concentrations in CIDR-GPG-modified ovsynch-treated group:
Plasma P4 concentrations were significantly higher (P<0.05) at day 4, and 7 in responsive group in comparison to those in non-responsive group (Figure 4a). Furthermore, the P concentrations in responsive group (8.85 ± 0.74 mg/dl) were significantly higher (P<0.05) in compare to those in non-responsive group (4.27 ± 0.07 mg/dl) (Figure 4b).
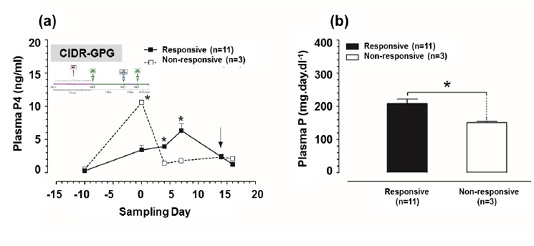
Figure 4: Plasma progesterone (P4) and plasma phosphorus (P) concentrations in CIDR-GPG-modified ovsynch-treated group. (a) Plasma P4 concentrations for both responsive and non-responsive groups. Zero (0) means day of first dose GnRH administration, and black arrow indicates day of PGF2α injection. (b) Plasma P concentrations for both responsive and non-responsive groups throughout the period of study. Asterisk means a significant difference at P<0.05. All values are presented as mean ± SEM.
DISCUSSION
Different therapies for treatment of postpartum anestrous buffaloes were previously studied (Karen and Darwish, 2010; Rahman et al., 2012; Terzano et al., 2012; Waqas et al., 2016; Sharma et al., 2017). The present study describes the reproductive response of anestrous buffaloes with inactive ovaries to ovsynch or modified-ovsynch regimens during the low-breeding season (from May to October).
In the present study, a significantly higher conception rate 57.1% reported in GPG-ovsynch treated buffaloes. The high efficacy of GPG-ovsynch protocol to synchronize ovulation in 78-90 % of treated buffaloes (Paul and Prakash, 2005) and to increase the conception rate in 60% of treated buffaloes in the high-breeding season (Baruselli, 2001) was previously reported. Other studies reported a lower efficacy when the ovsynch regimen was applied with a conception rate about 30.7-33.3 % (Paul and Prakash, 2005) and 34.6-34.9 % (Chaikhun et al., 2010). Such variation in the response to ovsynch regimen is likely attributed to differences in breed, condition of the ovaries, the quality of straw used in the AI and environmental conditions (season). The effect of seasonal variation is supported by Baruselli et al. (1999) findings reported different responses of the treated buffaloes to the GPG-ovsynch with conception rates of 48.8 % and 6.9 % during the short-day and long-day seasons, respectively. Different response to GPG-ovsynch with high conception rate (56.5 %) during the high-breeding season (de Araujo et al., 2002), and lower conception rate (36.0 %) if used during the transition period from short day to long day season. Ovsynch using GnRH and PGF2α could successfully synchronize the ovulation in both cyclic and a cyclic buffalo (Ali and Fahmy, 2007) and that the response was lower when the treatment was performed during the low-breeding season (Perera, 2008). Such lower pregnancy rate in buffaloes is also reported when the ovsynch protocol is randomly employed regardless the ovarian condition (Neglia et al., 2003). Presence of a large follicle at the beginning of ovsynch (time of first GnRH) is a determining factor for the successful synchronization of ovulation and high conception rates (de Rensis et al., 2005; Presicce et al., 2005).
Our study reported higher conception rate in response to CIDR-GPG compared to G-CIDR-PG regimen. It worthily noted that, CIDR insertion in the G-CIDR-PG is useful to prevent the premature estrus and increasing conception rates (Steckler et al., 2002). Moreover, CIDR has been effectively used to treat anestrous buffaloes (Singh, 2003a). Variable conception rates from 20 to 50 % had been previously reported in response to CIDR treatment (Hattab et al., 2000; Barile et al., 2001; Neglia et al., 2003; Drost, 2007; Azawi et al., 2012 a, b, c). Recently, a study using pluriparous Egyptian buffaloes reported that G-CIDR-PG modified ovsynch protocol increased both the follicular and post-ovulation luteal blood flow and improved the fertility indices, as well (Samir et al., 2019). Collectively, our current results and previous data indicate that P4 treatments result in satisfactory conception rates in buffaloes during both the low- and high-breeding seasons. It should be noted that, a recently published meta-analysis study reviewed 32 articles reported that ovsynch and different modified ovsynch estrus synchronization protocols have higher pregnancy per artificial insemination in cyclic compared with non-cyclic buffaloes (Du et al., 2021). Therefore, more studies are required to confirm the efficacy of P4-induced treatment in the postpartum anestrus in buffaloes (de Rensis and López-Gatius, 2007).
Significantly higher P4 concentration at the day PGF2α in the responsive buffaloes in the ovsynch or CIDR-plus modified ovsynch protocols could be predictor of probability of pregnancy as previously reported (Bello et al., 2006). Moreover, buffaloes responded to ovsynch regimen during low-breeding season had P4 concentration ≥ 2.0 ng/ml at the time of PGF2α (Jabeen et al., 2013). Generally, the increased levels of P4 increases the sensitivity of the hypothalamic-pituitary system (Singh, 2003a, b, c), which improves the intensity of heat. From the hormonal point of view; anoestrus buffaloes with P4 concentration below 1.0 ng⁄ml (Das and Khan, 2010) was considered responsive to ovsynch or modified-ovsynch protocols when the plasma P4 concentrations is more than 2.0 ng/ml after the 1st GnRH injection.
Plasma P concentrations throughout the study showed no change between responsive and non-responsive groups after GPG-ovsynch treatment, similar results had been previously reported by Hedaoo et al. (2008) who observed no change between serum P in cyclic or anoestrus buffalo. Modification of the ovsynch protocol by insertion of CIDR after the first GnRH injection in the G-CIDR-PG modified ovsynch treated group fails to increase the P concentrations in both the responsive and non-responsive groups throughout the whole period of sampling. Significantly higher plasma P concentrations in the responsive group compared to the non-responsive CIDR-GPG treated group would be a good indicator of improved reproductive performance, the physiological involvement of P in cAMP synthesis could be a pathway for its great influence on reproduction (Hurley and Doane, 1989). This notion is supported by the previous study that reported higher P concentrations in the cycling in compare to non-cycling buffaloes Newar et al. (1999). Moreover, our research group previously suggested that the CIDR-GPG would be a novel modified ovsynch estrus synchronization protocol in postpartum anoestrus dairy cows with high conception rate (Ahmed et al., 2017).
Our study suggests that the usage of CIDR before applying the GPG synchronization regimen could be more beneficial in improving the conception rates in buffaloes. Since the animals may utilize more P and other metabolites in activating the follicular dynamics and ripening the conceiving oocyte in the ovulatory follicle.
CONCLUSION
This study concluded that GPG-ovsynch synchronization protocol would be clinically beneficial for synchronization in postpartum anestrus buffaloes because of its better synchrony and improved fertility, in addition to, its lower cost in compare to other modified ovsynch protocol. There is a possibility that CIDR-GPG modified ovsynch protocol would improve the fertility, since the significantly higher P levels can be utilized in activating the follicular dynamics and maturation of the oocyte. More clinical studies are required to generalize the use of either GPG-ovsynch or CIDR-GPG modified ovsynch protocol in buffaloes.
ACKNOWLEDGEMENT
Authors would like to appreciate their deep thanks to staff members of Theriogenology, Obstetrics, and Artificial Insemination Department, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt for their help, scientific comments and support.
ETHICAL APPROVAL
All experimental protocols and procedures were in accordance to the Animal Ethics and Use Committee of the South Valley University, Egypt.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
authors contribution
A.S.A.H., A.M.I., N.A.M.Y., and A.E.A.: Prepared conception and design of study. A.M.I., and A.E.A.: Conducted the field study, examination of buffaloes, and blood sampling. A.E.A., A.S.A.H., A.M.I., and N.A.M.Y.: Collected laboratory samples and conducted biochemical analyses. A.M.I., A.E.A., A.S.A.H.: Manipulate and statistically analyzed the data. A.S.A.H., and A.E.A.: Drafted the manuscript. A.S.A.H., N.A.M.Y., and A.E.A.: Carried out final writing, critical review and revision. All authors have read and approved the final manuscript.
REFERENCES





