Advances in Animal and Veterinary Sciences
Research Article
Neoplasms In Equine Species In Sharkia Governorate, Egypt
Abdelmoneim A. Ali, Nahla AG. Ahmed Refat, Mohamed M.M. Metwally,
Mohammed S. Sobh*
Pathology Department, Faculty of veterinary medicine, Zagazig University, Sharkia, Zagazig 44159, Egypt.
Abstract | Few studies and very limited published data concerning the spread of neoplasia among Equidae in Egypt are available, therefore, the present study was carried out to investigate the prevalence and types of neoplasia among Equidae in Sharkia Governorate, Egypt, and to assess the efficacy of some available biomarkers as a diagnostic tools. This work was investigated 35 clinical cases of equine neoplasms (23 donkeys, 10 horses, and 2 mules) out of 1513 total examined cases admitted from different clinics in Sharkia Governorate, from May 2019 to October 2020. Depending upon gross and histopathological findings; the neoplastic growths include 12 cases of sarcoids, 9 cases of fibroma, 4 cases of squamous cell carcinoma, 3 cases of papilloma, 2 cases of fibropapilloma, 1 case of fibrosarcoma,1 case of myxoma, 1 case of of myxosarcoma, 1 case of myxoid liposarcoma, and 1 case of ovarian teratoma. Immunohistochemically, cytokeratin5 (CK5), MDM2, and S100 biomarkers were used to confirm the diagnosis of certain neoplasms. The Overall prevalence of equine neoplasms was 2.31 %. The diagnosed cases were linked with animal species and sex and the significance (p < 0.05) of differences among age, seasons, and location of the neoplasms were evaluated. The prevalence rates in domestic donkeys, horses, and mules were 2.66 %, 1.94%and 1.22% respectively. The rate of affected animals over 3 years was (3.38 %) in comparison with animals less than 3 years (0.19 %). Females (2.64%) were more affected than males (2.28%) and mules (1.42%). The prevalence rate was 5.56% in summer, 1.6 % in spring, 1% in autumn, and 0.85 % in winter. A higher rate of neoplasms was reported in extremities and trunk (1.25%), 0.52% in the head, neck, 0.19% back, ventrum, 0.19% in eye, related skin, 0.13 % in genital organs.
Keywords | Egypt, Neoplasms, Equine, Histopathology, Immunohistochemistry.
Received | August 12, 2021; Accepted | August 18, 2021; Published | October 01, 2021
*Correspondence | Mohammed Salah Sobh, Pathology Department, Faculty of veterinary medicine, Zagazig University, Sharkia, Zagazig 44159, Egypt; Email: mohamedsobh89@yahoo.com
Citation | Ali AA, Refat NAGA, Metwally MMM, Sobh MS (2021). Neoplasms in equine species in Sharkia Governorate, Egypt. Adv. Anim. Vet. Sci. 9(11): 2005-2013.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.2005.2013
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Sobh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Equine neoplasia leads to economic losses which ranged from undesirable effects on the animal’s skin to locomotors and reproductive dysfunctions. Equine sarcoid is considered to be one of the most prevalent skin affections in equids (Chambers et al., 2003). It represents nearly 20% of equine tumors and about 90% of equine skin neoplasia. Although it had a low mortality rate, it has a direct effect on the material value of horses and may lead to lameness according to their locations (Goodrich et al., 1998).
(Valentine, 2006) recorded the most common neoplasms in equine populations as the following; sarcoid (51.4%), squamous cell carcinoma (SCC) (18.3%) beside melanocytic tumors, papillomas, and mast cell tumors were, constituting (17.8%). while, lymphoma, fibrosarcoma, fibroma, schwannoma, hemangiosarcoma, lipoma, malignant melanoma, hemangioma, basal cell tumor, adenoma, and mammary adenocarcinoma respectively constitute the remaining percentage of neoplasm cases (12.5%). Sarcoid was the most common in horses and was the only common tumor in donkeys and mules. While, ocular SCC was prevalent in paints and quarter horses, and penile SCC was most common in appaloosas and quarter horses.
In Egypt, there is little published data about the prevalence of equine neoplasms, but few surveys as by (Hassanein and Mahmoud, 2009) diagnosed the most common affected neoplasms of equine species in different localities of Egypt (Assiut, El-Menia, and Sohag) which were 32.1 % sarcoids and 1.48% fibroma in 202 tumors collected from different farm animals.
Sarcoid developed in head and neck (39%), extremities (35%), ventrum (26%), and any parts of the equine body that were susceptible to trauma (Knottenbelt and Kelly, 2000). The prepuce area, abdomen, flank, chest, and trunk together with wounds of castration and groins were also included (Carr et al., 2001). Lesions frequently occur at the sites of wounds or surgery as well as other areas that are frequently rubbed by horses to remove flies (Ghim et al., 2004).
Neoplastic biomarkers serve as a differential diagnostic tools for some undifferentiated cases (Teruya‐Feldstein, 2010) as Cytokeratin 5 (CK5) for squamous cell carcinoma (SCC) (Chu and Weiss, 2002), MDM2 (Avallone et al., 2016) and S100 (Doria-Torra et al., 2015) for liposarcoma.
Here we aimed to demonstrate the prevalence through morphological, histopathological studies and evaluate the efficacy of certain available biomarkers for neoplastic growths in addition to post-excisional follow up of some cases in Sharkia Governorate.
Materials and Methods
Animals
Neoplastic growths were recorded in 35 equine species (23 donkeys, 10 horses, and 2 mules) among 1513 reared in different localities in Sharkia governorate from May 2019 to October 2020. A full description data for each case was recorded concerning their onset time, age, sex, region, shape, size, and consistency.
Diagnosis
All cases were subjected to clinical and histopathological examination. The clinical examination of the affected animals helped in the preliminary diagnosis as it gives information about the different clinical types of sarcoid which confirmed using the clinical classification of (Knottenbelt, 2005). Clinical evaluation was based on lesions appearance and reporting correlated skin lesions as ulceration, scaling, alopecia, and exudation.
Histopathological examination
Total excisional biopsies were collected from all proliferative lesions. The tissue specimens were fixed in neutral buffered formalin 10% for 48 hours, dehydrated in ethyl alcohol, cleared in xylene, embedded in paraffin wax, sectioned into 5μm in thickness using automated microtome then stained with routine Hematoxylin and Eosin (H & E). Some special stains were applied as Masson’s trichrome for staining collagen in fibroma cases. Moreover, an Alcian blue stain was used to demonstrate mucin in myxoid liposarcoma case. (Suvarana et al., 2018).
Immunohistochemical examination
An immunohistochemical staining procedure was done according to (Ali et al., 2015), the avidin-biotin-peroxidase complex (ABC) method (Hsu et al., 1981) was used with primary antibodies as the following:
Cytokeratin 5 (CK5) monoclonal antibody(clone XM26, Thermo Fisher Scientific, USA, dilution 1:20, Product # MA5-12596) for SCCs,
Anti-MDM2 Mouse monoclonal antibody, (clone 2A10, Abcam, Cambridge, UK, 1/100 dilution, ab16895) for liposarcoma, Anti-S100 alpha Rabbit monoclonal, (EPR5250, Abcam, Cambridge, UK , 1:100 dilution, ab133519).
Paraffin sections (5 μm) were placed on charged slides, deparaffinized, hydrated then antigen retrieval was performed. After the sections were incubated with the primary antibodies overnight at 4 °C in a humidified chamber, slides were washed with phosphate-buffered saline (PBS) and incubated with the secondary antibody (Expose mouse and rabbit specific HRP/DAB detection kit, Abcam; Ready-to-use; Cat.#: ab80436) for 15 minutes at room temperature in a humidified chamber then rinsed with PBS. Staining was then performed using the DAB chromogenic agent for detection of immunoreactivity, counterstained with hematoxylin, dehydrated, and mounted in a synthetic medium.
Statistical analysis
All statistical operations were performed using SAS statistical analysis software system Package V9.1. Univariate logistic regression model was fitted through Maximum Likelihood (ML) procedure to assess the effect of independent variables [animal species, age, sex, season and locations of neoplasms], on the dependent dichotomous variable (prevalence of neoplasms). P-values of less than 0.05 are considered having a statistically significant association
Table 1: Summary about history of the collected cases
|
Neoplasm
|
Organ | No. of Animal species | Prevalence among confirmed cases | Mean Age | Sex | Season |
| Sarcoids | skin of forelimbs, hind limbs, head, neck, shoulders and back |
12 cases (8 Donkeys, 3 horses, 1 mule) |
34.28% |
5-9 years |
7males, 4 females, 1 mule |
9 cases in summer, 2 in spring, 1 in autum |
| Fibroma |
Periocular skin, skin of forearm, fore and hind limbs. ventral abdomen |
9 cases 5 donkeys , 4 horses)) |
20% |
5-8 years |
6 males 3 females |
5 cases in summer, 1 in spring, 2 in autumn, 1 in winter |
| Squamous cell carcinoma |
periocular , eyelid skin and penis |
4 cases (2 donkeys,1 horse, 1 mule) |
11.42% |
6-10 years |
2 males, 1 female, 1 mule |
3 cases in summer, 1 in spring, |
| Papilloma |
Head skin , at pastern joint, female genitalia
|
3 cases 2 donkeys ,1horse |
8.57 % |
3-5 years old |
2 males, 1 female |
2 cases in summer, 1 in spring,
|
| Fibropapilloma | Skin of limbs | 2 cases of donkey | 5.71% |
7 & 11 years |
One male & one female |
1 case in summer, 1 in spring, |
| Fibrosarcoma | Skin at fetlock | Donkey | 2.85% |
10 Years |
Male |
1 in autum |
| Myxoma | Skin of neck | donkey | 2.85% |
6 years |
Male |
1 case in summer |
| Myxosarcoma |
Skin at fetlock |
donkey | 2.85% | 7years | female |
1 in winter |
|
Myxoid liposarcoma |
Forearm region |
donkey | 2.85% |
8 years |
male |
1 case in summer |
| Teratoma | Ovary | Mare | 2.85% |
7 years |
Female |
1 in winter |
with studied traits.
Results and discussion
Our survey identified of 35 clinical cases of equine neoplasms out of 1513 total examined cases. Summary about the age, sex, breed, location, and season of each collected case was presented in (Table 1).
Depending upon gross, histopathological and immunohistochemical findings; our results revealed 12 cases of sarcoids, 9 cases of fibroma, 4 cases of SCCs, 3 cases of papilloma, 2 cases of fibropapilloma, 1 case of fibrosarcoma,1 case of myxoma, 1 case of myxosarcoma, 1 case of myxoid liposarcoma, and 1 case of ovarian teratoma in mare. These results partially agree with (Valentine, 2006). (Pascoem and summers, 1981) found that the most commonly occurring neoplasms in horses were sarcoids, papillomas and SCCs of the eye and external genitalia. In another report by (Jaglan et al., 2018) reported 4 cases of fibroma, one squamous cell carcinoma, and one papilloma in 6 clinical equine cases.
The detailed findings of recorded cases are described as follows:
1- Equine sarcoid: The most commonly recorded neoplasms in this study. According to the owners’ complaints, the lesion appeared spontaneously and increase its size with time. The tumor appeared single or multiple firm or fleshy warts-like lesions or ulcerated growth which appeared grayish-white in color with some hemorrhagic spots. According to gross features, the majority of cases were fibroblastic type which manifested by fleshy ulcerated surface and serum exudation (Fig.1a). In contrast, the verrucous lesions are characterized by their warty appearance with variable degrees of flaking and scaling of their surfaces (Fig.1b).
Histopathological characteristics of equine sarcoid include epidermal acanthosis and hyperkeratosis with long rete ridges into the dermis (Fig.1c) that confirmed by (Scott and Miller, 2003). The dermal layer contains immature fibroblasts running in a wavy pattern and picket fence formation (fibroblasts arranged perpendicular to the basement

Figure 1: (a-b): Gross picture of the fibroblastic type of sarcoid showing fleshy ulcerated surface (arrow) (1a). Verrucous lesions showing a warty appearance with variable degrees of flaking and scaling of their surfaces (arrow) (1b). (c-d): Photomicrograph of H&E stained equine sarcoid showing epidermal acanthosis, hyperkeratosis, and hyperplasia with long rete pegs (arrow) (bar 200µm) (1c) and picket fence formation (arrow) (bar 20µm) (1d).
membrane) (Fig.1d) that agreed with (Hewes and Sullins, 2009; Semieka et al., 2012). The most epidermal layers were ulcerated with necrosis and hemorrhages and infiltrated with neutrophils. The superficial dermis showed thrombosis, hemorrhage, and inflammatory cells infiltration mainly neutrophils, lymphocytes, macrophages, and eosinophils.
Epidermal acanthosis in sarcoids could be attributed to growth-promoting factors expressed by the neoplastic fibroblasts (Carr et al., 2001). Sarcoid progresses from a benign to an aggressive fibroblastic phenotype (Brostrom, 1995). (Zahra et al., 2019) found fibroblastic type in a large proportion (70%) followed by the mixed type (13.5%), nodular (4.7%), verrucous (5.4%), and occult type (4.7%). (Jones et al., 1996) suggested that all connective tissue tumors (fibroma, fibrosarcoma, and neurofibroma) in horses are forms of equine sarcoids. However, (Foy et al., 2002) reported that differential diagnoses for equine sarcoid include granulation tissue, granuloma, papilloma, fibroma/fibrosarcoma, cutaneous lymphoma, SCCs, habronemiasis, and staphylococcal folliculitis. Our records were covered these differential diagnoses through histopathological examinations of collecting cases.
2- Fibroma: The fibroma cases appeared over the skin mainly on the periocular region (Fig.2a), at the medial aspect of the forearm (Fig.2b), forelimb and hind limbs, and ventral abdomen. The cases appeared grossly as pedunculated polypoid mass or wart-like nodule or large nodular spherical subcutaneous masses. All cases from donkeys were firm in consistency and grayish-white in color with a wet bleeding surface. The case located at the ventral abdomen had an ulcerated surface with necrotic debris and fleshy consistency. Histopathologically, the tissue section revealed a capsulated nodule with whirls of fibrous connective tissue which run in all directions with abundant collagen fibers in-between (Fig.2c). Masson’s trichrome stain revealed a positive reaction of blue-greenish collagen fibers and dark brown nuclei (Fig.2d).
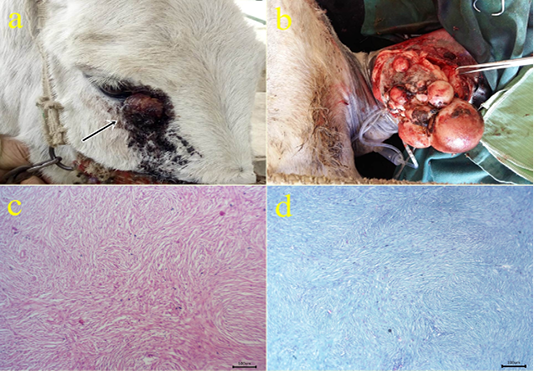
Figure 2: (a-b): Gross picture of fibroma showing nodular raised mass with the alopecic surface at periocular skin (arrow) (2a) and Pedunculated polypoid mass on the skin at the medial aspect of the forearm (1b). (c): Photomicrograph of H&E stained fibroma showing interlacing bundles of spindle-shaped fibroblast cells containing collagen fibers. (bar 100µm) (d): Photomicrograph of Masson’s trichrome-stained collagen of fibroma. (bar 100µm).
3-Squamous cell carcinoma (SCC): The neoplastic masses were lobulated growth (Fig 3a), solid in consistency and yellowish red in color. Microscopically, the tissue sections showed exophytic and endophytic growths with the dermal neoplastic invasion of pleomorphic epithelial cells (Fig.3b) with or without keratinized pearl formation and obvious mitotic figures surrounded by inflammatory cells. (Fig.3c). Immunohistochemical reactions revealed cytoplasmic expressions within the epithelial forming keratin pearls by cytokeratin 5 (Fig.3d). Similar results obtained by (Chu and Weiss, 2002). Cytokeratin was expressed in case of tumor progression and carcinoma (Frohwitter et al., 2016).
4- Papilloma: The cases were seated at head skin, caudal aspect at pastern joint (Fig.4a), and female genitalia. The lesions appeared as nodular grayish-white firm mass. Histopathologically, the tissue section revealed hyperkeratosis, acanthosis with finger-like papillae (Fig.4b) and spongiosis of the epidermal layers in addition to parakeratosis with remnant nuclei at the cornified layer.
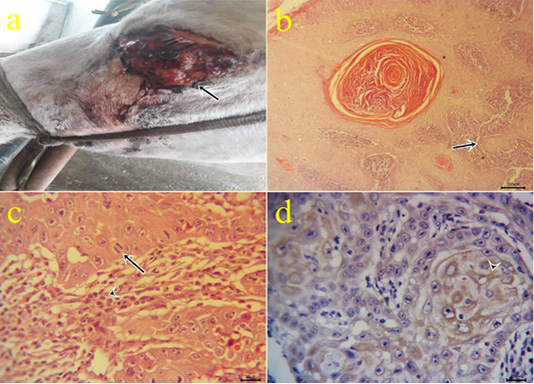
Figure 3: (a): Gross picture of SCC in male donkey showing Cauliflower-like mass at periocular and eyelid skin (arrow). (b-c): Photomicrograph of H&E stained SCC showing nests of neoplastic epithelial cells (arrow) (bar 100µm) (3b), obvious mitotic figures (arrow) surrounded by lymphocytes (arrowhead) (bar 20µm) (3c). (d) Positive cytoplasmic expression of CK5 within the invaded epithelium (arrowhead) of IHC counterstaining with Mayer’s hematoxylin (bar 20µm)

Figure 4: (a): Gross picture of papilloma in male horse showing white firm mass at the caudal aspect of pastern joint (arrow). b: Photomicrograph of H&E stained papilloma showing hyperkeratosis and acanthosis with finger-like papillae (arrowhead). (bar 200µm)
5-Fibropapilloma: Wart-like masses were seen on limbs (Fig.5a). Microscopically, the masses revealed hyperkeratosis and acanthosis with hypercellularity of dermal fibroblasts in the form of whirls (Fig.5b).
6-Fibrosarcoma: One case of fibrosarcoma located at the skin of the fetlock region. The mass appeared as a large firm nodule with a grayish red ulcerated surface (Fig.6a) and a whitish lobulated fleshy mass on the cut section. Microscopically, the growth was composed mainly of neoplastic fibroblasts that arranged in herringbone-like patterns with pleomorphic cells (Fig.6b), high mitotic index, angiogenesis, and tumor giant cells.
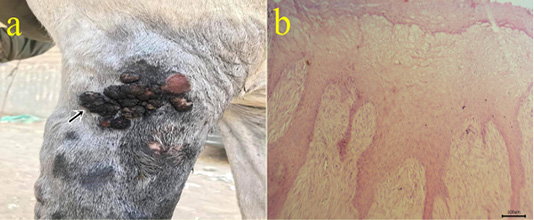
Figure 5: (a): Gross picture of fibropapilloma showing wart-like masses at the lateral aspect of arm region (arrow). (b): Photomicrograph of H&E stained fibropapilloma showing hyperkeratosis , acanthosis with hypercellularity of dermal fibroblasts in the form of whirls (bar 100µm).
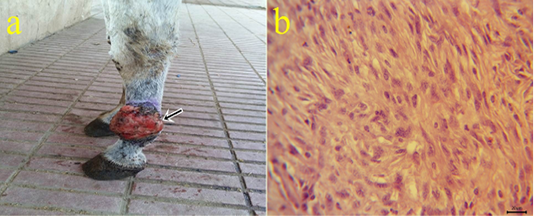
Figure 6: a: Gross picture of fibrosarcoma showing grayish red elevated mass with an ulcerated surface at the skin of the fetlock region (arrow). b: Photomicrograph of H&E stained fibrosarcoma showing pleomorphic fibroblasts with whirls (bar 20µm).
7-Myxoma: One case of myxoma was seen in the skin of the neck (Fig.7a). The mass appeared as solid, raised grayish-white color with dark red hemorrhagic spots on cut sections. The neoplasm is characterized microscopically by capsule formation (Fig.7b) with different shapes of embryonic white fibroblasts with interlacing processes and secretions (Fig.7 c,d).
8- Myxosarcoma: One case of myxosarcoma was found on the skin near to fetlock joint. The neoplastic mass was solid, slimy, and grayish-white color (Fig.8.a). The nodule exhibited microscopic characteristics of malignancy criteria, high mitotic index, giant cells (Fig.8b), hemorrhagic areas (Fig.8c), and cytoplasmic processes.
9- Myxoid liposarcoma: Large glistening neoplastic mass at forelimb region with the slimy cut surface (Fig.9a), the content of the growth comprised pleomorphic cells which ranged from the spindle, polygonal, oval cells which embedded in a mucoid substance containing poorly differentiated fat cells with undetectable collagen contents and variable sizes giant cells (Fig.9b) and limited mitotic index. Abundant capillaries with hemorrhage and hemosiderosis were also seen. Special staining with alcian blue revealed positive bluish myxoid background Fig.9c. Immunohisto-

Figure 7: a: Gross picture of myxoma showing a solid neoplastic mass on the skin of the neck in the male donkey. Fig.(7b-7d): Photomicrograph of H&E stained myxoma showing capsulated neoplasm (arrow) (7b), different shapes of embryonic fibroblasts with processes and faint basophilic secretions (7c-7d). (bar 200,100,20 µm) respectively.
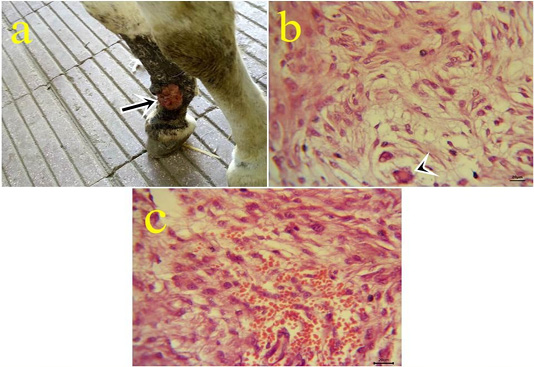
Figure 8: a: Gross picture of myxosarcoma showing solid, ulcerated grayish neoplastic mass at the fetlock joint in donkey (arrow). Fig.(8b-8c): Photomicrograph of H&E stained myxosarcoma showing pleomorphic cells and giant cells (arrowhead) (8b) (bar 20µm). Hypercellularity of embryonic fibroblasts with hemorrhage (8c) (bar 20µm).
chemical reactions revealed positive nuclear immunostaining with MDM2 (Fig.9d) and S100. In which, the genetic abnormalities can lead to specific nuclear protein overexpression of MDM2 that was previously detected by (Avallone et al., 2016). (Doria-Torra et al., 2015) applied an S100 biomarker to rule out a fibrosarcoma and confirmation of liposarcoma which give positive nuclear and cyto plasmic expressions.

Figure 9: a: Gross picture of myxoid liposarcoma showing large glistening slimy neoplastic excised mass from forelimb. Fig.(9b): Photomicrograph of H&E stained myxoid liposarcoma showing poorly differentiated fat cells (arrows) embedded in mucoid substance with undetectable collagen contents and variable sizes giant cells (bar 20µm). Fig.(9c): positive bluish myxoid background (arrow) using alcian blue staining (bar 20µm). Fig.(9d): MDM2 positive nuclear expression within the giant cells (arrowhead) of IHC counterstaining with Mayer’s hematoxylin (9d). (bar 10µm)
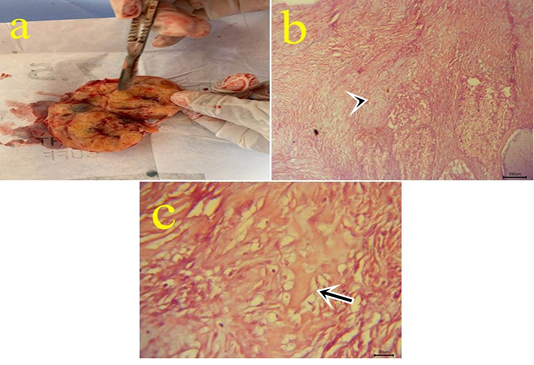
Figure10: a: Gross picture of teratoma showing enlarged firm yellowish excised ovarian mass from 7 years old mare. Fig.(10b-10c): Photomicrograph of H&E stained teratoma showing chondrocytes (arrowhead) (10b) (bar 100µm) and bone spicules (arrow) (10c). (bar 20µm)
10- Ovarian teratoma: In a 7-year-old mare with an enlarged firm yellowish rounded ovarian mass (Fig.10.a) with difficulty on cut sections. Microscopically, this mass is formed from tissues like cartilage, bone, and skin with ovarian tissue (Fig.10.b,c). Teratomas have been seen in the bitch, sow, mare, and cow and most of them are well-differentiated and benign (Jubb et al., 2007). Moreover, (Hassanein and Mahmoud, 2009) reported malignant teratoma in a cow ovary weighing 10kg. Ovarian teratoma might be
Table 2: Summary for main gross and microscopic lesions for different neoplasms in equine
|
Neoplasm
|
Gross picture | Main microscopic picture | Post excisional follow up (6 months later) |
| Sarcoids |
Ulcerated and hemorrhagic fibrous growths |
acanthosis, hyperkeratosis and picket fence formation, hemorrhage, necrosis, inflammatory exudate of epidermis |
4 cases recur 2 non follow up 6 not recur |
| Fibroma |
Large polypoid or nodular masses
|
Whirls of fibroblasts |
1 cases recur 2 not followed 7 not recur |
| Squamous cell carcinoma |
Cauliflower mass |
Cell nests within dermal layer |
3 cases recur 1 not recur |
| Papilloma |
Wart like |
hyperkeratosis, acanthosis | 3 cases not recur |
| Fibropapilloma |
Wart like |
hyperkeratosis, acanthosis, whirls in dermis | 2 cases not recur |
| Fibrosarcoma |
Huge ulcerated fibrous mass |
Whirls of fibroblasts with criteria of malignancy | Recur |
| Myxoma | Ulcerated nodule |
Embryonic white fibroblasts with processes and secretions. |
Not recur
|
| Myxosarcoma |
Large ulcerated nodule |
cytoplasmic processes of white fibroblasts, malignancy criteria and hemorrhages | not followed |
|
Myxoid liposarcoma |
large glistening neoplastic growth |
mucoid substance containing poorly differentiated fat cells |
|
| Teratoma |
Rounded mass, hard by cut section |
tissues other than ovarian tissue as cartilage, bone |
|
Table 3: Prevalence of equine neoplasms in sharkia Governorate, Egypt (May 2019 - October 2020)
| Risk factors | Examined cases (1513) |
Positive cases
(35) |
Prevalence % | OR | 95%CI |
P-value |
|
|
Donkeys |
Animal species | 862 | 23 | 2.66 | 1 | ||
|
Horses |
515 | 10 | 1.94 | 0.722 |
0.341- 1.530 |
0.3957 | |
|
mule |
136 | 2 | 1.22 | 0.544 |
0.127- 2.336 |
0.4132 | |
|
Less than 3 years |
Age | 508 | 1 | 0.19 | 1 | ||
|
over 3 years |
1005 | 34 | 3.38 |
17.752** |
2.423-130.052 | 0.0046 | |
|
Male |
Sex | 920 | 21 | 2.28 | 1 | ||
|
female |
453 | 12 | 2.64 | 1.165 |
0.568- 2.389 |
0.6771 | |
|
Mule |
140 | 2 | 1.42 | 0.620 | 0.144-2.675 | 0.5221 | |
|
Summer |
Seoson | 413 | 23 | 5.56 | 1 | ||
|
Spring |
375 | 6 | 1.6 |
0.276** |
0.111-0.685 | 0.0055 | |
|
autumn |
375 | 4 | 1 |
0.183*** |
0.063-0.534 | 0.0019 | |
|
Winter |
350 | 3 | 0.85 |
0.147*** |
0.044-0.492 | 0.0019 | |
|
Fore,hindlimbs,shoulders |
Locations |
1513 |
19 |
1.25 | 1 |
|
|
| Head,neck |
8 |
0.52 |
0.418* |
0.183-0.958 | |||
| Back,ventrum |
3 |
0.19 |
0.156*** |
0.046-0.529 | |||
| Eye,related skin |
3 |
0.19 |
0.156*** |
0.046-0.529 | |||
| Genitial oragns |
2 |
0.13 |
0.104*** |
0.024-0.448 | |||
OR= Odd ratios and * Significant differences.
seen due to hormonal disturbances and leads to a reduction in reproductivity and economic losses.
Collectively, the macroscopic and microscopic pictures of all confirmed cases were recorded in (Table 2).
All equine species were susceptible to neoplasms but the prevalence rate in donkeys (2.66 %) was higher than horses (1.94%) and mules (1.22%), the previous results come in agreement with (Yu, 2006). This could be due to exposure of donkeys to bad managemental conditions during drafting in different villages of Sharkia governorates. (Valentine, 2006) reported that the only common reported neoplasms in donkeys were sarcoids. Moreover, Quarter horses and Arabians have a high risk for sarcoid development. The higher incidence of sarcoid reflects higher exposure to bovine papillomavirus due to a large number of equine species were cohabited in places with bovine species with a higher density of predisposed animals or of viral vectors (Scott and Miller, 2003). Sarcoid neoplasms may cause discomfort due to continuous irritation and can result in ulceration, infection, and occasionally lameness associated with lesion location.
The prevalence rate was significant (p < 0.05) with age factor where equine over 3 years had prevalence rate (3.38%) higher than animals less than 3 years (0.19%).These might be due to a long time of exposure to different predisposing factors causing neoplasms. In the present study, the mean age of affected animals ranged from 6 to 8 years, although other studies found an incidence peak between 3 and 6 years of age (Knottenbelt, 2005). However, a gradual increase in incidence is observed up to the age of 15 years followed by a decrease in prevalence (Mohamed et al., 1992). Other researchers have reported a higher prevalence in younger individuals (Clottu, 2008).
The prevalence rate of diagnostic cases was linked with sex in which the prevalence rate of females (2.64%) was more than males (2.28%) and mules (1.42%). These could be attributed to exposed females to immunosuppressive agents as pregnancy, parturition, and lactation.
There was a significant association (p < 0.05) between seasonal variations and the prevalence of neoplasms, in which a high prevalence rate was detected in summer (5.56%) and lower rates in spring (1.6 %), autumn (1% ) and winter 0.85 %. Flies may play important roles in transmission of bovine papillomavirus and irritation of minor wounds to become sarcoids. This factor reflects the importance of fly control in sites where equids are living especially in summer seasons in Egypt farms.
The association between prevalence rate and the location of a neoplasm of equines was significant (p < 0.05), where extremities, trunk showed a higher prevalence (1.25%) than head,neck (0.52%), back,ventrum (0.19%), eye, related skin (0.19%) and genital organs (0.13 %). Our results are consistent with (Shokry et al., 2015) who reported that the most affected areas by sarcoids were in the limbs (40.9%), the paragenital and anal regions (22.0%), the periorbital (15.7%), the neck (11.3%), and other body areas (10.1%). Other studies by (Martens et al., 2001) reported the trunk and head are more affected than the extremities and (Zahra et al., 2019) who recorded most sarcoid lesions in the head region then forelimbs, hind limbs, belly, back, and perineal region. On the other hand (Foy et al., 2002) reported that common sites of sarcoid are lips, eyelids and ears. The possible explanation for the high prevalence of limbs, shoulders, head and neck lesions might be due to continuous injuries and irritation afflicted by bad harnesses or grooming tools.
The prevalence rates of recorded equine neoplasms were summarized in (Table 3).
In Conclusion, we present data on the morphological, histopathological features and the prevalence of equine neoplasms in sharkia governorates, Egypt and evaluate the efficacy of certain available biomarkers using immunohistochemistry technique in addition to post excisional follow up of some cases
Conflict of interest
The authors have no conflict to declare.
Authors contributions
AAA designed the study. MSS did the fieldwork, analyzed the data and prepared the draft manuscript. AAA, NAR and MMM guided, supervised and approved the final version of manuscript.
References





