Advances in Animal and Veterinary Sciences
Research Article
Comparison Between the Effect of Oral Administration of Alum and Aqueous Extract of Moringa oleifera Seeds on Physiological Liver Parameters of Male Albino Rats
Doaa Mohammed1*, Hanan Mahmoud2, Osama Ahmed3, Hamada Mahmoud4, Hanaa Fahim3, Heba Ahmed5
1Potable Water and Sanitation Company, Beni-Suef, Egypt; 2Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt; 3Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt; 4Biology Department, School of Sciences and Engineering, American University, Cairo, Egypt; 5Harmful Animals Department, Plant Protection Research Institute, Agriculture Research Center, Egypt.
Abstract | This study aims to assess the efficiency of Moringa oleifera aqueous seed extract as a natural coagulant in comparison with aluminum sulphate (alum) as a chemical coagulant and its effects on the liver of male albino rats. The animals were divided into 9 groups that were provided with 10% stock alum solution and 3 concentrations (2%, 5% and 10%) of aqueous Moringa oleifera seed extract as drinking water ad libitum for 2 periods 1 month and 2 months. Activities of various enzymes related to the liver including ALT, AST, ALP and LDH as well as albumin levels and total bilirubin concentration were investigated. Changes in liver oxidative stress and antioxidant defense system (LPO activity, liver GSH content, SOD, peroxidase (GPx) and transferase (GST) activities) were examined. Effect on mRNA gene expressions of P53, Bcl-2, TNF-α and IL-4 were tested. The most potent effect is 10% Moringa oleifera that causes a highly significant change in the second month in most parameters. The previous results indicated that aluminum (Al) is not a transition metal and cannot start per-oxidation. In conclusion, we found that long-term exposure of aqueous Moringa oleifera seed extract (MOSE) at 10% stock induces the accumulation of destructive phytocompounds. M. oleifera proved to be an effective water coagulating and antimicrobial agent for water purification, but the long-term treatment can produce harmful phytocompounds and induce stress on the cell.
Keywords | Natural coagulant, Seed extract, Antioxidant, Phytocompounds, Oxidation
Received | June 30, 2021; Accepted | July 01, 2021; Published | September 15, 2021
*Correspondence | Doaa Mohammed, Virology Department, Animal Health Research Institute (AHRI), ARC, Giza, Egypt; Email: elsaiedd@yahoo.com
Citation | Mohammed D, Mahmoud H, Ahmed O, Mahmoud H, Fahim H, Ahmed H (2021). Comparison between the effect of oral administration of alum and aqueous extract of moringa oleifera seeds on physiological liver parameters of male albino rats. Adv. Anim. Vet. Sci. 9(10): 1745-1756.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1745.1756
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mohammed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Water is the most essential natural resource for the sustainability of life on earth. About 70% of the human body weight is made up of water (Onifade and Ilori, 2008). It consequently acts an essential role in the composition and function of the human body (Tsuchiga and Utsumi, 2012; Eze and Ananso, 2014). Owing to random human activities, water quality has corrupted causing many diseases which plague the human race; therefore, access to safe and clean drinking water is a major concern throughout the world (Arora et al., 2013). Conventional methods are used to improves water quality. Drinking water treatment by aluminum sulphate (Al2 (SO4)3) (known as alum) is frequently used for decades through a coagulation process that improve the elimination of particulate, colloidal, and dissolved substances (Hadyer and Rahim, 2015). Aluminum concentration may increase in drinking water during the treatment process, if aluminum is overdosed or the water treatment process is dysfunctional (Meghzili et al., 2016). The residual aluminum may have some health effects on consumers (Driscoll and Latterman, 1995; Othmani et al., 2020). A Canadian study of health and aging claims showed an association between aluminum and neuropathological diseases including pre-senile dementia and Alzheimer’s disease (Jekel, 1991; Ohyagi and Miyoshi, 2013). Chlorine and chlorin-based disinfectants have been reported to have the potential for forming carcinogenic and mutagenic disinfectant byproducts (DBPs) such as tetrachloromethane (TCM) which produces hormonal analogue that interferes with male fertility (Science Dossier, 2017). DBPs may also be associated with cardiovascular disease, cancer and birth defects (Bichi et al., 2012). These restrictions make it necessary to find safe drinking water purification methods.
Developing countries used natural coagulants of plant source for years in water purification process because it is an uncomplicated, dependable and commercial method (Ida, 2013; Lakshmi et al., 2017). Moreover, the extracts from plant origin have both coagulating and antimicrobial properties and are safe for human health (Dalen et al., 2009; Megersal et al., 2014). Moringa oleifera (M. oleifera) is considered the most known cultivated species of a monogenic family; the Moringaceae (Nalamwar et al., 2017). The Moringa species are nowadays widely interesting due to their exceptional economic potential. Among these species, M. oleifera is the most commonly used for its nutritious and many medicinal products (Booth, 1988; Andy et al., 2008). Almost every part of the plant can be used for different purposes (Singh and Sharma, 2012; Nalamwar et al., 2017). The seeds of M. oleifera are the most commonly used natural coagulant in drinking water clarification that decreases the health risks related to excess turbidity (Lea, 2010; Zaid et al., 2019). In the rural context, the availability, acceptability and environmental safety of synthetic chemicals used in water purification must be ensured (Ince, 1989). Nonetheless, if effective, the use of natural biodegradable plant origin materials to bury turbid surface waters can be encouraged. In Africa and in south Asian countries, the Seeds of M. oleifera have been recommended for water treatment (Santos et al., 2005). The seeds of the Moringa family are, according to Ndabigengesere and Narasiah (1998), very effective water coagulants and have no toxic side effects (Gottsch, 1992). This study was therefore planned to evaluate the effects of aqueous extract of M. oleifera seed at a concentration of 2, 5 and 10% in comparison with alum at concentration of 10% on serum liver function markers (ALT, AST, ALP, LDH, total bilirubin and albumin), oxidative stress marker and antioxidant defense enzymes (LPO, GSH, SOD, GPx and GST), inflammation markers (TNF-α and IL-4) and apoptotic and anti-apoptotic markers (P53 and Bcl2) in the liver.
Material and Methods
Collection and Preparation of M. oleifera seed stocks
Dried seeds of M. oleifera were brought from Agriculture Research Centre, Egypt. The shell covering the seed kernels was removed manually, and the kernels were crushed into a fine powder using mortar and pestle (Kardam et al., 2010). Two grams of this powder were mixed for 3 minutes with 100 ml tap water. The solution was then stirred for 10 minutes using a magnetic stirrer, settled for 48 hours and eventually filtered with Whatman No.1 filter paper. The resulting stock solution had a concentration of approximately 1000 mg/L (2%). The previous step was repeated to prepare 5% and 10% stock solutions. By mixing 10 grams aluminum powder with 100 ml tap water, the aluminum sulphate (alum) stock solution was prepared. Throughout the experimental process, fresh stock solutions were prepared daily.
Experimental animals
In the present study, sixty adult male Wister strain albino rats, weighing 100-120 g, were used. The animals were obtained from the National Institute of Ophthalmology, Giza, Egypt. Before beginning the experiment they were kept under observation for two weeks to rule out any intercurrent infections. At normal temperature (20-25Co) and normal daily lighting cycle (10-12 hrs/day), the animals were housed in well aerated cages in Animal House of Zoology Department in Faculty of Science, Beni-Suef University, Egypt. Animals were given food (balanced standard diet) and water ad libitum. All animal procedures are in accordance with the general guidelines of animal care and the recommendations of the Experimental Animal Ethics Committee of Faculty of Science, Beni-Suef University, Egypt (Ethical Approval Number: BSU/FS/2015/18). All efforts were done to reduce the number and suffering of animals. The adult male albino rats were provided with water extract of the seeds of M. oleifera with varied stock concentrations (2, 5 and 10 %), which were given to animals as drinking water ad libitum. The procedure was repeated for alum solution with stock concentration (10%). Six rat representatives from each group were sacrificed after 1 month and 2 months.
Animal grouping
The adult male albino rats of this study were allocated into nine groups each contains 6 rats designed as follows:
Group I (Normal control 1group); rats of this group were administered with distilled water as a drinking solution for 1 month
Group II (alum-administrated group); animals within this group were administered 10% alum solution as drinking solution for 1 month
Group III (2%-administrated group treated with aqueous extract of the seeds of M. oleifera); this group was given 2% aqueous extract of the seeds of M. oleifera as drinking solution for 1 month
Group IV ( 5%- administrated group treated with aqueous extract of the seeds of M. oleifera); this group was given 5% aqueous extract of the seeds of M. oleifera as drinking solution for 1 month
Group V (10%- administrated group treated with aqueous extract of the seeds of M. oleifera); this group was given 10% aqueous extract of the seeds of M. oleifera as drinking solution for 1 month
Group VI (Normal control 2 group); the rats of this group were given distilled water as a drinking solution for 2 months
Group VII (alum-administrated group); as group 2, but 10% alum solution was given as drinking solution for 2 months
Group VIII (2%-administrated group treated with aqueous extract of the seeds of M. oleifera); as group 3, but 2% aqueous extract of the seeds of M. oleifera was given as drinking solution for 2 months
Group IX (5%- administrated group treated with aqueous extract of the seeds of M. oleifera); as group 4, but 5% aqueous extract of the seeds of M. oleifera was given as drinking solution for 2 months
Group X (10%- administrated group treated with aqueous extract of the seeds of M. oleifera); as group 5, but 10% aqueous extract of the seeds of M. oleifera was given as a drinking solution for 2 months
The rats were anesthetized at the end of the experiment by diethyl ether and blood samples were collected from the jugular vein and also, after cervical dislocation and dissection, the liver was subsequently excised and included in isotonic saline (0.9 % Nacl).
Blood and organ sampling
The blood samples were allowed to coagulate and serum was then isolated by centrifugation for 15 min at 3000 r.p.m., collected into sterilized tubes and stored at -2o C. One gram of frozen liver tissue was homogenized in 10 ml 0.9% NaCl to yield 1% homogenate (w/v). The homogenized liver was centrifuged for 15 min at 3000 r.p.m. Parts of 3 mm3 liver were stored at -70 oC in sterilized Eppendorf tubes for being used for RNA isolation and RT-PCR analysis. Activities of serum Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) were determined using reagent kits purchased from Biosystem S.A. (Spain) using the Gella et al. (1985) method. Alkaline phosphatase (ALP) activity in serum was determined using reagent kits purchased from BioSystem S.A. (Spain) consistent with the Schumann et al. (2011) method. Lactate dehydrogenase (LDH) serum activity was determined using reagent kits purchased from Spectrum, following the method of Zimmerman (1979). According to the method of Doumas et al. (1971), serum albumin concentration was determined using a reagent kit purchased from Diamond Diagnostics Chemical Company (Egypt). Serum total bilirubin concentration was determined according to the method of Jendrassik and Grof (1938) using reagent kits purchased from Diamond Diagnostics Chemical Company (Egypt). Liver lipid peroxidation (LPO) was estimated using Yagi (1987) process, and superoxide dismutase (SOD) activity was evaluated according to the Marklund and Marklund (1974) technique. Glutathione peroxidase (GPx) activity was calculated using Matkovics et al. (1998) test. The content of liver glutathione (GSH) was evaluated by the method of Beutler et al. (1963) with some modifications; GST activity in liver homogenate was tested with few modifications using the Mannervik and Guthenberg (1981) chemical process. Usage of Thermo Scientific Verso 1-Step RT-PCR ReddyMix kit (Applied Biosystems, Foster City, CA, USA) to analyze the mRNA expression of IL-4, P53, TNF-α and Bcl-2 using specific primers located in Table 1.
Statistical analysis
Analysis od data was performed using student ANOVA test and comparing between means using LSD (Least Significant Difference) at P< 0.05) as outlined by PC-STAT (1995).
Results
Effect on liver functions
Effect on serum activities of ALT, AST, ALP and LDH:
Table 2 illustrated the effect of the alum and the aqueous extract of M. oleifera seeds on the levels of various enzymes related to liver function including ALT, AST, ALP and LDH in albino rats. Concerning ALT activity, the data re
Table 1: Primer sequence used for qRT-PCR.
| mRNA species | primer sequence | Reference |
| IL-4 |
F: 5 d GGAACACCACGGAGAACG3´ R: 5 d GCACGGAGGTACATCACG3´ |
|
| P53 |
F: 5 d CAGCGTGATGATGGTAAGGA3´ R: 5 d GCGTTGCTCTGATGGTGA3´ |
|
| Bcl-2 |
F: 5 d GGGATGCCTTTGTGGAACTA3´ R: 5 d CTCACTTGTGGCCCAGGTAT3´ |
|
|
TNF-α |
F:5 GCTGAGGTTGGCGGATAAA3´ R:5 AAAATCCTGCCCTGTCACAC3´ |
(Sreeja et al., 1965) |
|
β-actin |
F: 5 dTCACCCTGAAGTACCCCATGGAG3´ R: 5 d TTGGCCTTGGGGTTCAGGGGG3´ |
Table 2: Effect of alum and M. oleifera seed aqueous extract on activities of various serum enzymes related liver function in albino rats.
|
Parameters
|
Groups |
ALT (U/l) |
AST (U/l) |
ALP (U/l) |
LDH (U/l) |
| First Month | Normal 1 |
32.2±1.49 b |
161.68±1.43 b |
85.98±6.99 de |
60.03±1.36e |
| 10% Alum |
24.8±1.49 c |
141.70±3.27 cd |
79.05±7.52 e |
71.96±1.12cd |
|
|
2% M. oleifera |
32.5±1.04 b |
158.8±6.1 b |
77.27±5.04 e |
111.40±2.37b |
|
|
5% M. oleifera |
24.2±1.04 c |
151.9±11.5 bc |
119.19±6.00 bc |
67.52±1.56de |
|
|
10% M. oleifera |
24.4±1.41 c |
134±3.69 d |
95.61±2.97 de |
62.89±7.36de |
|
| Second Month | Normal 2 |
31.7±1.16 b |
162.1±1.09 b |
83.54±3.41 e |
58.59±6.43e |
| 10% Alum |
26.7±1.92 c |
142.5±3.7 cd |
102.2±5. 1cd |
82.45±2.40c |
|
|
2% M. oleifera |
32±2.53 b |
148.3±3.19 bcd |
117.83±5.96 bc |
126.83±0.17a |
|
|
5% M. oleifera |
26.8±2.31 c |
135.5±3.69 d |
133.24±10 b |
73.82±5.61cd |
|
|
10% M. oleifera |
47.2±1.59 a |
197.5±4.83 a |
214.65±7.61 a |
67.00±0.16de |
|
| F-probability | P< 0.001 | P< 0.001 | P< 0.001 | P< 0.001 | |
| LSD at the 5% level | 4.82 | 15.24 | 18.42 | 10.96 | |
| LSD at the 1% level | 6.49 | 20.53 | 24.8 |
14.76 |
|
Data are expressed as mean ± standard error (SE). Number of rats in each group is six, for each parameter means, which share the same superscript symbols, are not significantly different at P < 0.05.
vealed that experimental rats treated with 10% alum and 2% extract in the two months produced a non-significant change (p>0.05; LSD) as compared to normal rats. Also, treatment with 5% of the extract showed a highly significant (p<0.01; LSD) decrease for the same periods. On the other hand, a highly significant (p<0.01; LSD) increase was seen in the group treated with 10% water extract of M. oleifera for the second month. Regarding serum AST activity, treatment with 2% aqueous extract of M. oleifera for the two periods and 5% aqueous extract for the first month induced a non-significant (p>0.05; LSD) change as compared with normal controls. Moreover, the treatment with 10% stock alum solution at both months produced a significant (p<0.05; LSD) decrease as compared with the corresponding normal control. The supplementation of 10% aqueous extract of M. oleifera at first month and 5% aqueous extract of M. oleifera at second month induced a highly significant (p<0.01; LSD) decrease as compared with the corresponding normal control. While treatment with 10% aqueous extract of M. oleifera produced a highly significant (p<0.01; LSD) increase for a second month. Regarding serum ALP activity, administration of the alum, 2% and 10% extract for one month induced a non-significant (p>0.05; LSD) change. On the other, the treatment with 5% extract for the first month and all the extract concentrations for the second month caused a highly significant (p<0.01; LSD) increase. The serum LDH activity exhibited a non-significant (p>0.05; LSD) change as a result of treatment with 5% and 10% aqueous extract of M. oleifera for a first month and 10% aqueous extract for a second month. At the first month, there was a significant (p<0.05; LSD) increase as a result of 10% alum stock. A highly significant (p<0.01; LSD) increase was recorded for treatment with 2% M. oleifera extract at the first and all the extract concentrations for the second month as compared to normal rats.
Table 3: Effect of alum and M. oleifera seed aqueous extract on serum albumin and bilirubin in albino rats.
|
Parameters
|
Groups |
Albumin (g /dl) |
Bilirubin (mg/dl) |
|
First Month |
Normal 1 |
3.34±0.15 a |
1.00±0.04 e |
|
10% Alum |
3.47±0.15 a |
1.36±0.08 cde |
|
|
2% M. oleifera |
3.45±0.13 a |
1.23±0.18 cde |
|
|
5% M. oleifera |
3.18±0.08 a |
1.24±0.12 cde |
|
|
10% M. oleifera |
3.18±0.06 a |
2.07±0.20 ab |
|
|
Second Month |
Normal 2 |
3.07±0.24 a |
1.17±0.01 de |
|
10% Alum |
2.85±0.20 a |
1.63±0.26 bc |
|
|
2% M. oleifera |
2.96±0.21 a |
2.11±0.20 a |
|
|
5% M. oleifera |
2.95±0.36 a |
1.61±0.11 cd |
|
|
10% M. oleifera |
3.09±0.25 a |
2.16±0.18 a |
|
|
F-probability |
P> 0.05 |
P< 0.001 |
|
|
LSD at the 5% level |
______ |
0.45 |
|
|
LSD at the 1% level |
______ |
0.61 |
|
Data are expressed as mean ± standard error (SE). Number of rats in each group is six, for each parameter means, which share the same superscript symbols are not significantly different at P < 0.05.
Table 4: Effect of alum and M. oleifera seed aqueous extract on lipid peroxidation and glutathione content in the liver of albino rats.
|
Parameters Groups |
Lipid peroxidation (n mole MDA/100 mg tissue/hr) |
Glutathione (n mole/100 mg tissue) |
|
|
First Month |
Normal 1 |
35.76±0.63 a |
12.71±0.80 c |
|
10% Alum |
29.48±6.67 a |
12.12±1.22 c |
|
|
2% M. oleifera |
18.46±1.77 b |
13.90±1.46c |
|
|
5% M. oleifera |
12.11±0.94 b |
34.49±2.28 b |
|
|
10% M. oleifera |
10.35±0.49 b |
42.43±5.99 ab |
|
|
Second Month |
Normal 2 |
32.28±0.86 a |
12.71±0.54 c |
|
10% Alum |
27.48±5.59 a |
15.10±2.27 c |
|
|
2% M. oleifera |
15.73±0.73 b |
15.72±2.18 c |
|
|
5% M. oleifera |
16.33±0.72 b |
47.68±3.53 a |
|
|
10% M. oleifera |
16.35±0.51 b |
44.40±6.03 a |
|
|
F-Probability |
P< 0.001 |
P< 0.001 |
|
|
LSD at the 5% level |
8.29 |
9.33 |
|
|
LSD at the 1% level |
11.17 |
12.56 |
|
Data are expressed as mean ± standard error (SE). Number of rats in each group is six, for each parameter means, which share the same superscript symbol are not significantly different at P < 0.05.
Effect on total albumin and bilirubin levels concentration
The obtained data showed the effect of the alum and the water extract of M. oleifera seeds on albumin levels and total bilirubin concentration in albino rats were expressed in Table 3. Albumin level demonstrated a non-significant (p>0.05; LSD) change for all treatment concentrations at both periods when compared with normal control rats. The supplementation of 10% stock alum solution and 2% M. oleifera extract at one month in addition to 5% M. oleifera seed extract for two periods exhibited a non-significant (p>0.05; LSD) change on total bilirubin concentration. On the other hand, a highly significant (p<0.01; LSD) increase was induced for treatment with 2% M. oleifera extract at the second month and 10% extract for two periods. Finally, 10% stock alum solution for the second month showed a significant (p<0.05; LSD) increase.
Effect on liver oxidative stress and antioxidant defense system
Effect on liver LPO activity and liver GSH content: Data regarding the changes in liver lipid peroxidation
Table 5: Effect of alum and M. oleifera seed aqueous extract on various antioxidant enzyme activities in the liver of albino rats.
|
Parameters
|
Groups |
SOD (U/g tissue) |
GPx activity (mU/100g tissue) |
GST activity (U/100mg tissue) |
| First Month | Normal 1 |
17.42±0.78 a |
53.19±3.71 b |
42.73±4.14 a |
| 10% Alum |
16.38±0.91 a |
60.41±6.49 b |
43.60±2.98 a |
|
|
2% M. oleifera |
18.35±0.51 a |
45.67±3.33 b |
44.69±3.83 a |
|
|
5% M. oleifera |
16.03±2.43 a |
51.96±4.62 b |
43.84±4.07 a |
|
|
10% M. oleifera |
13.92±1.97 a |
31.05±2.54 c |
37.88±4.43 a |
|
| Second Month | Normal 2 |
17.27±0.70 a |
56.72±3.31 b |
39.92±4.83 a |
| 10% Alum |
14.28±0.98 a |
54.09±3.87 b |
29.46±0.99 a |
|
|
2% M. oleifera |
15.18±1.43 a |
91.70±9.94 a |
27.93±1.72 a |
|
|
5% M. oleifera |
14.97±1.05 a |
98.46±8.54 a |
38.40±6.77 a |
|
|
10% M. oleifera |
14.98±0.77 a |
108.10±11.93 a |
36.93±4.25 a |
|
| F-Probability | P> 0.05 | P< 0.001 | P> 0.05 | |
| LSD at the 5% level | _____ | 19.04 | ____ | |
| LSD at the 1% level | _____ | 25.63 |
____ |
|
Data are expressed as mean ± standard error (SE). Number of rats in each group is six, for each parameter means, which share the same superscript symbols are not significantly different at P < 0.05.
(LPO) activity and glutathione (GSH) liver content in the albino rats treated with distilled water as a normal control; treated with 10% stock alum solution and those treated with different M. oleifera stock solutions are represented in Table 4. Data expressed liver LPO activity declared a highly significant (p<0.01; LSD) decrease for all treatments with aqueous extract of M. oleifera seed at both periods as compared with normal control. While a non-significant (p>0.05; LSD) change was produced as a result of 10% alum supplementation for both months. Regarding liver GSH content, the supplementation with 5% & 10% aqueous extract of M. oleifera seed extract caused a highly significant (p<0.01; LSD) increase for both months. Also, treatment with 2% M. oleifera extract and 10% alum possessed a non-significant (p>0.05; LSD) change for both periods as compared with normal control rats.
Effect on liver SOD, peroxidase (GPx) and transferase (GST) activities: Data concerning the effect of 10% stock alum solution and different M. oleifera stock solutions on superoxide dismutase (SOD) as well as glutathione peroxidase (GPx) and glutathione-s-transferase (GST) activities in the liver of normal rats are tabulated in Table 5. Data expressed liver SOD activity showed a non-significant (p>0.05; LSD) change for all treatments at both months as compared to corresponding control rats. Hepatic GPx activity exhibited a non- significant (p>0.05; LSD) effect at both months as a result of treatment with 10% alum and a significant (p<0.05; LSD) decrease at the first month as a result of treatment with 10% M. oleifera extract. While the supplementation of 2%, 5% and 10% M. oleifera extracts produced a highly significant (p<0.01; LSD) increase at the second month as compared to corresponding control rats. Similar to SOD activity, hepatic GST activity expressed a non-significant (p>0.05; LSD) change for all treatments at both months.
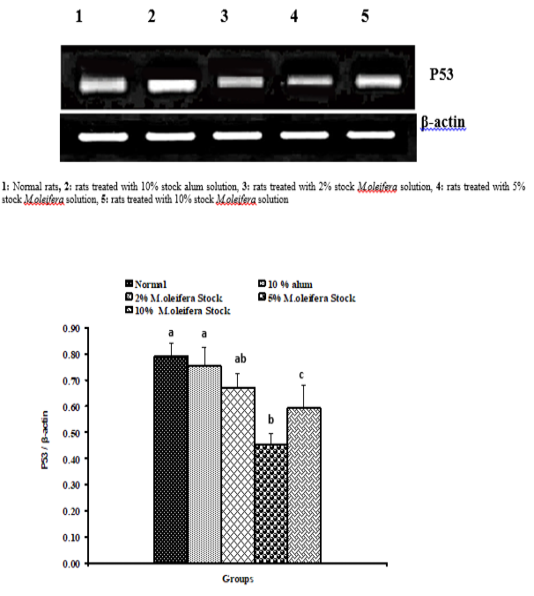
Graph 1: Effects of alum and M. Oliefera seed aqueous extract on liver P53 mRNA expression relative to β-actin of albino rats
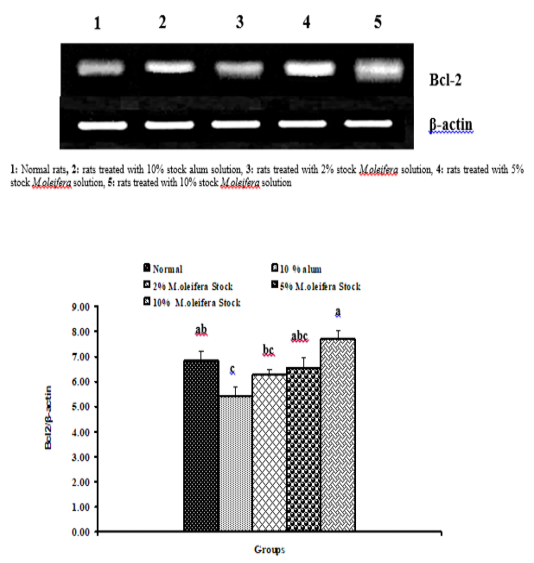
Graph 2: Effects of alum and M. Oliefera seed aqueous extract on liver Bcl-2 mRNA expression relative to β-actin of albino rats
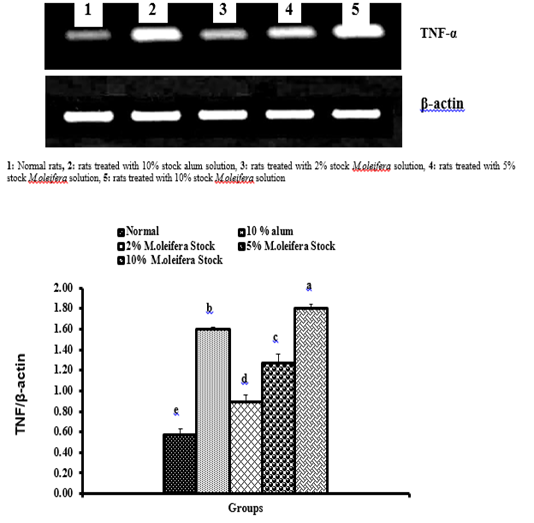
Graph 3: Effects of alum and M. oliefera seed aqueous extract on liver TNF-α mRNA expression relative to β-actin of albino rats
Effect on mRNA gene expressions of P53 and Bcl-2
The mRNA expression levels of liver tissue P53 and Bcl2 were shown in Graphs (1 & 2). Concerning P53 mRNA gene expression, the data showed a non- significant (p>0.05; LSD) effect as a result of treatment with 10% alum and 2% extract. While at the end of the experiment a highly significant (p<0.01; LSD) decrease was noticed as a result of 5% and 10% extracts’ supplementation. Bcl2 mRNA gene expression exhibited a non- significant (p>0.05; LSD) change as a result of treatment with 2%, 5% and 10% M. oleifera extract and a significant (p<0.05; LSD) decrease for 10% alum supplementation.
Effect on mRNA expressions of TNF-α and IL-4
Data described the effects of alum and different moringa stocks on mRNA expression levels of liver tissue TNF-α and IL-4 were depicted in Graphs (3 & 4). Compared to the normal control, treatment with 2%, 5% and 10% M. oleifera extract significantly (p<0.05; LSD) affected TNF-α mRNA gene expression. Concerning IL-4 mRNA gene expression, a highly significant (p<0.01; LSD) decrease was observed in all groups as compared to normal control rats.
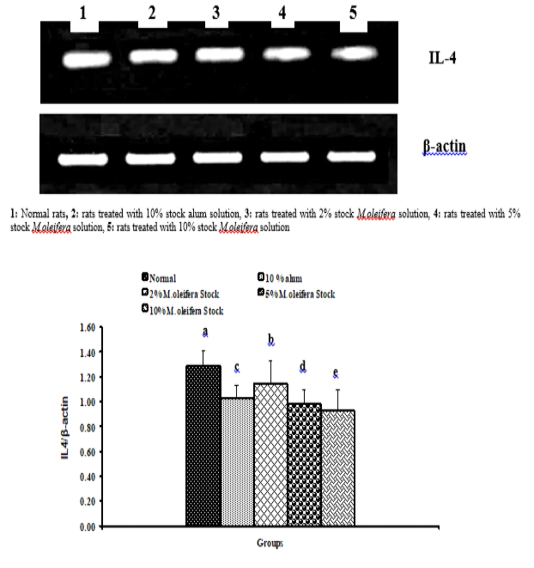
Graph 4: Effects of alum and M. Oliefera seed aqueous extract on liver IL-4 mRNA expression relative to β-actin of albino rats
Discussion
Effect on biochemical parameters
Enzyme activity changes are considered to be indicators of stress throughout the physiological status of lives (Lal Shah, 2010). In our study, high levels of ALT and AST serum values are associated with supplementation of 10% aqueous extract of M. oleifera seed for 60 days; as well as in ALP activities of all treatments for a long period. This may be related to the liver tissue damage caused by modifications of the membrane components in the tissue, follow-on discharge of these enzymes into the blood thus, elevation in serum enzyme activities level. While ALT and AST serum values recorded a decrease in animals treated with stock alum solution and low concentrations of M. oleifera extract. This decrease of AST and ALT may cancel the toxic effects, keeping the structure of the liver cell membrane and maintain the balance of the body against toxins (Lohner et al., 2001). Also, Bharali et al. (2003) noticed that the oral supplementation of the hydroalcoholic extract of M. oleifera improved levels of hepatic enzymes involved in the detoxification of xenobiotic substances. This result suggests hepatoprotective properties of preparations of M. oleifera seeds, which in line with the decrease in hepatic enzymes as a result of treatment with low concentrations of M. oleifera extract in our study. This cellular protection is due to the presence of Z-carotene in drumsticks of the plant, a precursor of vitamin A (Geervani & Devi, 1981). In both periods the elevation in LDH activity has been seen in all groups. The increase in the LDH activity may be attributed to cardiac leakage as a result of necrosis induced by alum and low dose of M. oleifera extract. High doses of M. oleifera extract lower the activity of LDH, which may be due to its operation to preserve membrane integrity and thus limit the release into circulation of these cardiac markers (Radhiga et al., 2012). Bilirubin is a product of the degradation of the heme constituent of the hemoglobin molecule. In animals with a hemolytic anemia, overall serum bilirubin is elevated, and this increase is caused mainly by an increase in the indirect reacting bilirubin. The level to which bilirubin in hemolytic anemia is elevated depends on the destruction rate of red cells and the liver’s ability to excrete newly formed bilirubin (Tripathi, 2003).
Effect on oxidative stress and antioxidant defense system
LPO has been comprehensively used as an indicator of oxidative stress (Huggett et al., 1992). Cellular defense systems including antioxidant enzymes, such as CAT, SOD, GPx, and non-enzymatic scavengers, such as glutathione, vitamin A and E protect the organism against oxyradical damage such as DNA, strand breaks, protein oxidation and the induction of lipid peroxidation (Winzer et al., 2000). Under normal healthy conditions, a balance is maintained between oxidative stress and antioxidant requirements. Our experiment indicated a non-significant difference of liver LPO in rats treated with 10% stock alum solution for both months. Coagulant alum is used to neutralize colloidal particulate (Zeta potential) charges and organize larger flocks that can be eliminated in the distribution system. Alum dissociates into aluminum ion and sulphate ion when added to the water. Several diseases have been linked with aluminum (Al3) (which is a highly reactive element and pervasive ecological pollutant) has been associated with some (Fulgenzi et al., 2014). Although aluminum is not a transition metal and cannot initiate per-oxidation, a correlation between Al accumulation and oxidative damage in different organs has been sought in many studies. On the other hand, the results of this research show that the administration of aqueous M. oleifera extract at 2%, 5% and 10% stocks for both terms in the liver shift the homeostasis towards a less free radical rich oxidizing state. These findings are attributed to the phytocompounds which can bring harmony against any derangement of metabolism. Bioactive phytocomponents of seeds are efficient radical scavengers and possess persuasive reducing capability (Mantena et al., 2009). SOD, GPx, GSH and GST constitute a mutually supportive team of defense against ROS. SOD is a metalloprotein and is the first enzyme involved in the antioxidant defense by lowering the steady level of O2- (McDonald et al., 1991). Our results showed a non-significant change of SOD levels during alum and moringa exposure in liver for both periods. This is due to the metabolic stress by alum or excessive harmful phytochemicals in M. oleifera seed extract did not develop yet. GPx scavenges the highly reactive lipid hydroperoxide in the aqueous phase of cell membranes (Rister and Banchner, 1976). Oxidative damage induced by alum and long-term administration of different M. oleifera stocks resulted in stimulation of GPx activity. GSH is a main non-protein thiol in living organisms, which acts as a central function in sharing the body’s antioxidant protection process. Perturbation of GSH status of a biological system can lead to serious consequences (Campos et al., 1989). The results of the present research showed that aluminum as a pollutant induces antioxidant system and M. oleifera acts as a bifunctional inducer as it induces both phase-І and phase-II system enzymes that furnish the balance of xenobiotic metabolism towards detoxification. GST symbolizes a complex grouping of proteins. It has different functions and participates in many types of reactions. Most of these enzymes can catalyze GSH conjugation with compounds containing an electrophilic center by forming a thioether bond between the GSH sulpher atom and the substrate (Chasseaud, 1979; Mannervic, 1985). As well, a number of GST isoenzymes show additional GSH-dependent catalytic behavior including the decrease of organic hydroperoxides (Ketterer et al., 1990) and isomerization of different unsaturated compounds (Benson et al., 1977; Jakoby and Habig, 1980). These enzymes as well have numerous non-catalytic functions that convey the sequestering of carcinogens, intracellular transport of a broad spectrum of hydrophobic ligands, and modulation of signal transduction pathways (Cho et al., 2001). It was found that GST increased in the first period and after that decreased in the liver for alum and various stocks of MOSE but decreased in the remaining tissues for both periods. Stress developed due to alum or harmful phytochemicals in MOSE may lead to exhaustion of antioxidant defense pool by lowering the levels of antioxidant enzymes like GST and/or by increasing ROS production.
Effects on liver mRNA expressions of proinflammatory and apoptotic markers:
Tumor suppressor genes are natural genes that decelerate cell division, fix DNA mistakes, or notify cells when die (a process known as apoptosis or programmed cell death). Any disruption in tumor suppressor genes function makes the cells grow out of control leading to cancer. Many different tumor suppressor genes have been known, including TP53 (p53), BRCA1, BRCA2, APC and RB1 (Abreu Velez and Howard, 2015). The p53 tumor suppressor gene is the most obvious between these because mutations have been confirmed in a part of roughly every tumor type tested. p53 status analysis may be considered as a tool for predicting effective therapeutic regimens, whereas p53 itself, especially mutant p53, may show cancer therapy targets (Rivlin et al., 2011). A study by Daniela et al. (2002) showed that oxidative stress in the fibroblast cells of Alzheimer patients mediates DNA damage-induced apoptosis via p53-independent pathway. In our study, non-significant change was detected for alum and 2% M. oleifera seed extract administration and a significant decrease was observed for 5% and 10% M. oleifera seed extract. These results collectively suggest that the observed downregulation of p53 protein in alum and various M. oleifera seed extract stocks treated cells may not be posttranslational regulated, but transcriptional down-regulation is responsible for the observed effect. Our results show that the apoptotic pathway caused by treatments in rat neuroblastoma cells is p53- independent. TNF-α, a cytokine that plays a role in many inflammatory diseases, is produced by several pro-inflammatory cells (mainly macrophages, but also monocytes, dendritic cells, B-cells, CD+4 cells, neutrophils, mast cells and eosinophils) and structural cells (fibroblasts, epithelial cells and smooth muscle cells). TNF-α is a well-known inducer of the inflammatory response and a regulator of immunity. Its inflammatory properties are classically mediated through a wide variety of pro-inflammatory cytokines, including IL-1, IL-2, IL-6, IL-12, IFN-γ (interferon-γ) and TGF-β (transforming growth factor-β), generated mainly through NF-kB activation (Almogren, 2011; Günal et al., 2011). The elevation of TNF-α level was observed in all groups. Aluminum contact enlarged the increase in hippocampus TNF-α, a proinflammatory mediator. The increased hippocampus TNF-α stimulates microglia to discharge glutamate, which causes excitotoxicity (Tsunoda and Sharma, 1999; Takeuchi et al., 2008). Significantly elevation in TNF-α levels than controls in response to MOSE stocks this is reliable with a previous report by (Mahajan et al., 2009), this additional illustrates an anti-inflammatory role for Moringa. While TNF-α is a cytokine typically accompanied with pro-inflammatory responses, divergent roles for TNF-α have been stated whereby there are instances where it also has an immunosuppressive function. Dysfunctional adipocytes are found to be more receptive to TNF-α signaling and subsequent apoptosis, associated with macrophage infiltration and secretion of inflammatory cytokine (Subramanian and Chait, 2009). Apoptosis is an active process of cellular destruction with distinctive morphological and biochemical features. Two major apoptotic pathways have been defined in the mammalian cells; the death receptor and the mitochondrial pathways as mentioned by Pfeiffer and Singh (2018). The Bcl-2 family proteins are the most important regulators of the mitochondrial pathway. Signals from the death receptor pathway could be also bridged to the mitochondrial pathway via the Bcl-2 family proteins (Banta et al., 2018). In our study, the data showed a significant decrease of anti-apoptotic marker (Bcl-2) in rats treated with alum when compared with control. These findings cooperatively recommended that alum may cause activation of mitochondrial pathway of apoptosis. The changed Bax/Bcl-2 ratio is serious to Al-induced apoptosis (Johnson et al., 2005) causing activation of caspase-3 and release of cytochrome c (Savory et al., 2003). Sharma and Mehra (2008) declared decreased Bcl-2 expression and elevated proapoptotic marker (Bax) expression in the rat hippocampus. Kumar et al. (2009) stated that aluminum induces neuronal cells oxidative stress and increases p53 protein expression via activating p38 MAPK to start apoptosis and this is accompanied by a marked inhibition of Bcl-2 and increased Bax expression. In this study, M. oleifera extract induced down-regulation in Bcl-2 expression compared to the levels of controls. It was shown similarly that one of the essential components by which capsaicin, zerumbone, matrine and leptin anticipate HCC involves a decrease in the proportion of Bcl-2/Bax (Ma et al., 2008). Under those lines, M. oleifera seed extract initiated apoptosis by down-regulation of Bcl-2 and Bcl-xL and up-regulation of Bax and caspase-3 could be one of the essential mechanisms of HCC averting. Interleukin-4 is a pleiotropic cytokine produced largely by activated Th2-polarized T-cells, mast cells and basophils. It acts upon a broad range of targets, including hematopoietic cells, endothelial cells and tumor cells (Galizzi et al., 1990). Our study demonstrated a decrease in IL-4 levels in all groups. Many studies showed that IL-4 plays an important role in learning and memory processes (Gadani et al., 2012). Nolan et al. (2005) stated that there is a link between decreased IL-4 and long-term potentiation impairment.
Acknowledgments
The authors sincerely acknowledged all members of zoology department, Faculty of Science, Beni Suef University.
Conflict of interest
The authors declare that they have no competing interests.
Authors Contributions
Hanan M. Sayed participated in writing the manuscript. Hammada M. Mahmoud shared in revising the manuscript. Osama M. Ahmed proposed the research pan, guided the experimental work and shared in writing and revising the manuscript. Hanaa I. Fahim revised the manuscript and also supervised the experimental work. Heba Y. Ahmed shared in writing and revising the manuscript.
References
Int. J. Mol. Sci. 19:1-10. https://doi.org/10.3390/ijms19020448