Advances in Animal and Veterinary Sciences
Review Article
Platyhelminths Glycoconjugates in Diagnosis and Immune Response of Farm Animals
Eman E. El Shanawany*
Department of Parasitology and Animal Diseases, National Research Centre, El Bhooth St, Dokki, post pox 12622, Giza, Egypt.
Abstract | Platyhelminths (Flatworm: Neodermata) are a highly varied group of parasites containing several veterinary, biomedical and economic importance. Glycans of platyhelminths are expressed in a varity of carrier molecules including O- and N-linked glycoproteins and glycolipid structures. Their glycans exist in large quantities in the excretory/secretory products and on the parasites surface, these glycans play role in host-parasite interplay. Glycan portions of complex molecules of platyhelminths interact with antigen-presenting cells of the innate immune system, such as interact with specific receptors on dendritic cells leading to modulate host toward T helper 2 immune response for helping parasite survival. The immunomodulatory properties of platyhelminths glycans could be used to treat autoimmune and inflammatory diseases. The adaptive immune response against them characterized by the production of IgG, IgE, IL-4, IL-5, and IL-13. Anti-glycan antibodies are a common characteristic in platyhelminths infection, detection of these antibodies is widely used for serodiagnosis of platyhelminths infection. The Platyhelminthes glycoproteins and glycolipids are considered immunodiagnostic candidates for serodiagnosis of such infection in different farm animals. The specificity and sensitivity of diagnostic platyhelminths glycans are depending on the structural characterization of these glycans. Hence, the aim of this review will revolve around the application of these glycans for diagnosis of infectious diseases in farm animals. Understand their role in shaping and modulating innate and adaptive host immune responses. Immune modulation of host immune response by these glycans will make these molecules show promise in the fields of diagnosis, treatment of inflammatory and auto immune diseases, and vaccinology.
Keywords | Platyhelminthes, Glycans, Farm animals, Diagnosis, Immunomodulatory properties
Received | March 17, 2021; Accepted | May 26, 2021; Published | August 25, 2021
*Correspondence | Eman E. El-Shanawany, Department of Parasitology and Animal Diseases, National Research Centre, El Bhooth St, Dokki, post pox 12622, Giza, Egypt; Email: ee.elshanawany@hotmail.com
Citation | El-Shanawany EE (2021). Platyhelminths glycoconjugates in diagnosis and immune response of farm animals. Adv. Anim. Vet. Sci. 9(10): 1692-1704.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1692.1704
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 El-Shanawany. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Platyhelminths (Flatworm: Neodermata) are phyla contain a highly diverse group that have soft body surrounded by a plasma membrane or tegument (Mathison and Pritt, 2018). The platyhelminths phylum consists of four classes: Turbellaria (planarians), Monogenea, Cestoda (tapeworms), and Trematoda flukes (Littlewood, 2008). The classes turbellaria, monogenea don’t parasitized the farm animals (Zhang et al., 2019). The other two classes of platyhelminths are cestodes, and trematodes have veterinary and economic importance worldwide (Littlewood, 2006), infect both human and farm animals including cattle, camel, buffaloes, sheep, goat, and pig causing a significant economic loss (Bamaiyi, 2012). Most platyhelminths parasites have multi-host life cycles, including a vertebrate definitive host and one or more vertebrate or invertebrate intermediate hosts. The epidemiology of platyhelminths (cestodes and trematodes) is worldwide as they have wide geographical distribution in different regions especially America, Middle East, Asia and Africa (Saratsis et al., 2019).
So, the preparation of the cheap and efficient diagnosis of these infections in farm animals is a crucial issue. An extensive research effort has been focusing on the identification of protein antigens of the major platyhelminth species in the quest for immunogenic structures that could be used as vaccines and diagnostic components, with little success (Raina et al., 2011; González-Sapienza and Cachau, 2017). Currently, some attention turned to characterize glycans of platyhelminths as an alternative strategy (Haslam et al., 2001). Platyhelminths express a wide range of glycan structures, mainly on their secreted products, and the dominant antibody responses elicited in infected hosts offer promising prospects for the advancement of glycan-based diagnostics (Rodriguez et al., 2015).
Attention to glycans of platyhelminths was increasing as contributors to immunomodulatory properties of platyhelminth antigens as they switch the host immune responses toward T helper 2 (Th 2) (Rodríguez et al., 2017a). The Th2 responses were distinguish by secretion of interleukin (IL)-4, IL-9, IL-5, and IL-13 (Maizels et al., 2012) with anti-glycan IgM and IgG response. Also, mast cell, eosinophilia, and IgE were increased. The immunomodulatory properties of immune response against glycans of platyhelminths suggest that they may be used to improve vaccines. Hütter and Lepenies (2015) and diagnosis (Veríssimo et al., 2019), and can be used as therapy for autoimmune disorders (Maizels et al., 2018).
The glycome of Schistosoma (S) mansoni is by far the best-known, with data on glycan structural information now available for the parasite’s entire life cycle (Smit et al., 2015), however, the veterinary important S. bovis did not pay the same attention. Also, other platyhelminths glycan and have been analyzed, for example, N- and O-glycan in Fasciola (Abdel-Rahman et al., 2016; Garcia-Campos et al., 2016), in Echinococcus (Del Puerto et al., 2016), and in Taenia (Cruz-Rivera et al., 2019), and still there is need to deep study for glycans of these economic important platyhelminths. However, for most other veterinary important platyhelminths parasitic glycomic information is as yet largely incomplete.
Hence, this review will highlight the glycan structures that are synthesized by selected helminth species belonging to the platyhelminths phyla, and the potential role of these glycans in the diagnosis of platyhelminths infection in farm animals. Moreover, understand the role of platyhelminths glycans in innate and adaptive immunity.
Glycans overview
Glycans: Glycans or carbohydrates are one of the major biomolecules composed of hydrogen, carbon, and oxygen. Recently intensive attention has been focused on glycans biology and biochemistry for many reasons. First, the functional significance of glycans has been elucidated in metabolism and various biological processes in prokaryotic and eukaryotes such as recognition, signaling, and interaction between cells, fertilization, replication of the virus, and host- pathogen relation (Fincher, 2009). Second, the importance of glycans especially those attached with proteins (glycoproteins) in medicine, the next generation of drugs and vaccines will come from recombinant proteins. In some cases, there are some researchers discussed that recombinant proteins form are non-functional, for these reasons glycosylation of proteins may prevent this problem (Geldhof et al., 2007).
Glycoconjugates are formed when glycans linked to proteins (glycoprotein) or to lipids (glycolipid). Biosynthesis of glycan is a share in the process of glycosylation. Sugars are transmitted into cells through the plasma membrane or are saved at lysosomes from degraded glycoconjugates. Activation of sugar generally takes place in the cytoplasm. After activation, sugar nucleotides are transported into the endoplasmic reticulum/Golgi apparatus and used by different glycosyltransferases. The glycosyltransferases and other glycan-processing enzymes play a remarkable role in glycosylation of glycan (Spiro, 2002). They also, specify the final structure of glycans secreted or found on the cell surface, results in formation of glycocalyx (Rodriguez et al., 2015). Glycoproteins properties were determined by the structure of glycan, and site of glycan linkage to protein (Cipollo et al., 2005; Varki, 2017). Protein glycosylation can be divided into two types O- and N-glycosylation. For N-glycosylation, the N-acetylglucosamine (GlcNAc) residue is bound to an asparagine (Asn). In O-glycosylation Ser/ Thr residues of mucin and mucin-like proteins are first attached to a residue of N-acetylgalactosamine (GalNAc) (Haslam et al., 2001).
Platyhelminths glycomics: Characterization and identification
Glycomics is studying the entire component of sugar which found free or in a complex molecule of an organism, include physiologic, genetic, and pathologic (Aoki-Kinoshita, 2008). Also, glycomics means to study the structure of organism glycan or any cell type and is a branch of glycobiology. The term glycomics is come from the chemical prefix for sugar, “glyco-”, and was developed to follow the omics suffix defined by genomics (which deals with genes) and proteomics (which deals with proteins) (Rudd et al., 2015). The glycomic approaches were helping in the discovery of novel antigenic glycans use as glycol vaccines or diagnostic tools (Rodriguez et al., 2015).
Glycan can be purified by using high-performance liquid chromatography and ion-exchange chromatography immobilized with lectin. Lectin affinity chromatography is preferable used early in the purification scheme immobilized on a solid matrix (Cummings et al., 2017). Lectins are protein bind specifically to carbohydrates, they offer many advantages for the isolation of glycoconjugates: (1) Lectins are often cheap to buy and are in many cases also simple to purify. (2) Lectins have a high binding capacity and stable in a variety of conditions. (4) Elution from lectin columns can be highly selective by employing competing for mono, di-, or oligosaccharides (Fukuda and Kobata, 1993; O’Connor et al., 2017).
Detection of platyhelminths glycans and or antibodies against them is important for the diagnosis of platyhelminths infection in different hosts including farm animals. Glycan and antibodies against them were done by different tools include; lectin microarray, ELISA types assay, cell selection, cell agglutination, cell sorting, and histochemistry. The lectins, antibodies or carbohydrate-binding molecules (CBMs) were used in these assays (Cummings et al., 2017). Using a combination of lectins and antibodies printed on a slide to assess the glycosylation status of cells and glycoconjugates is one of the most common applications of lectins, CBMs, and antibodies. When it comes to deciding whether biological samples vary in glycosylation, this method is particularly sensitive (Hirabayashi et al., 2013). Each of the antibodies, lectins, and CBMs has different benefits. Lectins are usually less expensive than antibodies (Cummings et al., 2017).
Diagnostic application of platyhelminths glycoconjugates
Based on (1) large number of different glycan structures found in excreted/ secreted products and on platyhelminth’s surface. (2) dominant antibody responses against these glycans in infected hosts. It was suggested that these glycans can give advantage for development of glycan-based diagnostics by detecting generated antibodies against these glycans and their glycoconjugates in urine or serum of host (Restrepo et al., 2000; Van Die and Cummings, 2010a).
Here, the potential use of most veterinary important platyhelminths glycan antigens for serodiagnosis of cestodes infection (Echinococcus and Taenia), and trematodes infection (Fasciola) will be reviewed.
Cestodes (Tapeworm infection)
Taenia: In developed countries, the cysticercosis diagnosis is depending on clinical manifestation, nuclear magnetic resonance imaging finding, and computed tomography. The main drawback for using imaging techniques, however, is the possibility to overlook the infection, especially with a low parasites load and/or the figures are not standard (Millogo and Njamnshi, 2019). Also, these techniques need high cost this result to make these techniques are ineffective in diagnosis of cysticercosis in farm animals especially in developing countries (Zobba et al., 2014). The development of an immunodiagnostic test, which detects specific antibodies in serum or cerebrospinal fluid is, therefore, an important issue because the simplicity and reliability of these tests (Ito and Craig, 2003). Glycoproteins from Taenia spp. have long been used for serodiagnosis (Cruz-Rivera et al., 2019). Species-specific glycan profile was expressed by different Taenia species. T. solium oncosphere was rich in D-glucose, D-manose, D-galactose, and D- GalNAc residues, while D-GlcNAc residues were present in smaller ratio with the absence of N‐acetylneuraminic acid, and fucose residues (Arana et al., 2013). Also, these glycan compositions have been recorded in T. solium metacestode tegument (Alvarez et al., 2008). It has been reported that on the membrane surface in T. solium cysticerci there are, an additional residue, sialic acid (Landa et al., 2010). The Tn glycan was present in T. hydatigena (Freire et al., 2003). The Man5−9GlcNAc was reported in both T. crassiceps (Lee et al., 2005) and T. solium (Restrepo et al., 2000), by using western blot technique the researchers confirmed that T. solium Man5−9GlcNAc structures antigens are potent in cysticercosis diagnosis in both human and pigs (Restrepo et al., 2000) by immunohistochemistry (Obregón-Henao et al., 2003). Ito et al. (1999) purified glycoprotein faction from T.solium and proved its potency in the diagnosis of swine cysticercosis using ELISA as it was able in differentiating infected pigs from uninfected ones. Moreover, Sato et al. (2006) purified glycoproteins of cyst fluid from 2 genotypes of T. solium and used ELISA to prove its potency in the diagnosis of swine cysticercosis. Cruz-Rivera et al. (2019) succeeded in finding the expression of the 3 glycoproteins (TS14, T24H, and GP50) in different parts of the adult tapeworm T. solium. By using western blotting techniques, these glycoproteins are found to be specific in neurocysticercosis diagnosis. The same authors revealed that TS14 and T24H glycoproteins have a possible role during the development of the parasite, where at the early stages of proglottid maturation they are expressed in sexual organs. GP50 glycoprotein, on the other hand, was not expressed by any of the sperm cells in the seminiferous tubules at various stages of differentiation. The fact that highly immunogenicity of analyzed glycoproteins, combined with evidence that they are differentially expressed during sperm cell formation, indicates that they may play a functional role in fertility; thus, further research is needed to assess their potential for vaccine development. Rodriguez et al. (2012) reviewed that total homogenate of T. solium metacestodes contains seven antigenic glycoproteins namely GP50, GP42-39, GP24, GP21, GP18, GP14, and GP13 which purified by lentil-lectin chromatography. Anyone of these glycoprotein fractions was proved its potency in the diagnosis of porcine cysticercosis with initial sensitivity and specificity 98 and 100%, respectively. Hancock et al. (2004) discussed that the GP50 antigen of T. solium is a diagnostic marker for cysticercosis by western blot methods with 100% specificity and 90% sensitivity. Moreover, GP50 glycoproteins are diagnostic marker for T. multiceps infection in naturally infected goats and sheep by indirect ELISA with 95% sensitivity and 92.6% specificity (Huang et al., 2016). In different ways for diagnosis of bovine cysticercosis is the detection of circulating glycoprotein antigen of an execratory secretory product of T. saginata metacestodes in cattle using IgM monoclonal antibody-based ELISA with 93.4% specificity (Van Kerckhoven et al., 1998).
The few studies that conducted to study composition and immunological properties of Taenia spp. glycoconjugates have been focused to glycoproteins (Rodriguez et al., 2012; Cruz-Rivera et al., 2019). Little studies have been conducted on non-protein carbohydrate-containing structures of Taenia spp. However, in many other pathogenic parasites glycolipids are considered an important immunomodulating factors and valuable diagnostic markers (Yamano et al., 2006). The Galα1–4Gal and Galα1– 6Gal sequences have been demonstrated in glycosphingolipids isolated from T. crassiceps (Dennis et al., 1993). Baumeister et al. (1992) determined the chemical and serological staining patterns of glycolipids from three taeniid species, T. crassiceps, T. solium and T. saginata metacestodes. The glycolipid of T. solium structure was identified as family of β-galactosylceramides composed mainly of phytosphinganine (2-hydroxylated sphinganine). Serologically, the patterns of such glycolipids exhibit the same immunological reaction against normal mouse serum, infected sera and a monospecific polyclonal antibodies directed against T. crassiceps trisaccharide. The results didn’t confirm that this glycosphingolipid alone did not be able to use as diagnostic marker for neurocysticercosis, more investigations were necessary to study its use as diagnostic antigen for disease in cerebrospinal fluids merits (Lopez-Marin et al., 2002).
Echinococcus Spp.:WHO Informal Working Group on Echinococcosis (WHO-IWGE) approved the use of ultrasound in the diagnosis of Cystic Echinococcosis (CE) and Alveolar Echinococcosis (AC) and classification of the cyst (WHO, 2003), but this diagnosis should be completed by serology. CE and AC serological diagnosis are usually based on the detection of antibodies against hydatid fluid antigen but it reveals several drawbacks, like false negative and positive results and detectable antibody level, was long persistence in cured animals. Search about the alternative diagnostic candidate to improve test performance and standardization is an essential issue. Khoo et al. (1997) proved that E. granulosus hydatid cyst wall and protoscoleces are characterized by N- glycosylated structures of. The E. granulosus laminated layer is an extracellular mucin-rich matrix consisted of T antigen (Galβ1-3GalNAc), and Tn antigen [Galβ1-3 (GlcNAcβ1-6) GalNAc] (Díaz et al., 2009). The T antigen,s Galβ1-3 also coated with additional residues of Galβ1-3, Galβ1-3 glycans elongation play role in differentiatiation between E. granulosus and E. multilocularis (Del Puerto et al., 2016). Khoo et al. (1997) proved that E. granulosus antigen 5, Ag5, consists of a single polypeptide chain that is thereafter dissociated into single disulfide 22 and 38 kDa subunits have a N-glycan decorated with up to two phosphorylcholine and one core α1,6-fucose residues. The 22-kDa component has a highly conserved glycosaminoglycan-binding motif that may help to retain Ag5 in the host tissue around the parasite (Lorenzo et al., 2003). Subsequent analysis for Ag5 using mass spectrometry, other N-glycans was identified, and containing two α1,6-fucose core antennas with phosphorylcholine (PC) cap (Paschinger et al., 2012). Ag 5 is one of the most widely used immunodiagnostic antigens for CE in camel and sheep (Al-Olayan and Helmy, 2012). Koizumi et al. (2011) succeeded in the synthesis of glycan antigen Em2 (11) which is isolated from E. multilocularis hydatid cysts laminated layer and proved 95% sensitivity in the diagnosis of hydatidosis. El Shanawany et al. (2019) succeeded in isolating three glycoprotein antigens from the hydatid cyst germinal layer which is GlcNAc, Gl and Gal and proved that the GlcNAc fraction was the best to diagnose the CE in camel with 97.3 % sensitivity and relatively low specificity 54.5%. Kamel et al. (2006) used Con A purification method to develop a sensitive and specific candidate for a diagnosis of CE in humans and camel using ELISA with 96.9% sensitivity and high specificity (98.4%). A similar Con A purification method followed by subsequent gel filtration chromatography was used to isolated glycoprotein fraction from E. multilocularis and designed as Emgp-89 which proved serodiagnostic performance for canine infection with 83% specificity (Kouguchi et al., 2011).
The neutral glycolipid fraction’s major carbohydrate epitope of E. granulosus was the same as or very similar to that of T. crassiceps neutral glycol (sphingo) lipids, as represented by the ‘neogala’-series core structure (Dennis et al., 1993). The neutral glycosphingolipid fraction (Galb1-6Gal) was isolated from crude metacestodes extract and show antigenic properties by using ELISA (Persat et al., 1991, 1992). The glycolipids have the advantage to be synthesized chemically and use it in serodiagnosis. Four glycosphingolipid molecules of E. multilocularis were synthesized by Yamamura et al. (2004), their serodiagnostic potential was studied and one of them, Galb1-6(Fuca1- 3) Galb1-6Galb1-ceramide, proved potency in differentiation between AC and CE (Yamano et al., 2006).
Trematodes
Fasciola Spp.: Carbohydrate moieties from parasites like Echinococcus (Paschinger et al., 2012; Lin et al., 2013), S. mansoni (Frank et al., 2012; Lindholz et al., 2018) among others (Van Die and Cummings, 2010 a, b) have been well determined, however, glycoconjugates generated by Fasciola still need more studies. Researchers have identified reactivity of miracidia, redia and sporocysts surface (Georgieva et al., 2012) and gut of adult flukes (Farahnak et al., 2010; McAllister et al., 2011) with lectin this explains the presence of Glc and Man, GlcNAc or GalNAc residues. F. hepatica adult stage contains many types of glycan structure like, LDN [LacdiNAc (GalNAcβ1,4GlcNAc], fucosylated LDN, LDNF, [GalNAcβ1,4(Fucβ1-3)GlcNAc], truncated O-glycans, known as T antigen (Galβ1-3GalNAcβ1-O-Thr/Ser) LDN and LDNF (Nyame et al., 1998; Van Die and Cummings, 2010a). Using lectin blot Ravidà et al. (2016) proved that F. hepatica adult worm tegument was glycosylated with mainly oligomannose oligosaccharides that are found in its suckers, spines, and tegument coat. Moreover, Tn (GalNAc-O-Ser/Thr) antigen was found in F. hepatica adult worm. The Tn antigen was expressed mainly in testis, while in the parenchyma, the basal membrane of tegument and the apical membrane of the digestive tube epithelial cells were expressed sialyl-Tn glycoproteins (Freire et al., 2003). Also, Abdel-Rahman et al. (2016) isolated similar Tn antigen from adult F. gigantica with structure was O-glycan associated with protein sequences Ser-(Arg-Ser-Arg-Ser) and this glycoprotein proved potency in the diagnosis of bovine fascioliasis. Ghosh et al. (2005a) separated a 27 kDa glycoprotein antigen from F. gigantica adult worm, using Indirect ELISA the anti-27 kDa antibodies were detected in goat sera at the 2nd-week post-infection without cross-reaction with Paramphistomum epiclitum infected goat sera. Ghosh et al. (2005b) also used 27 kDa glycoprotein in early diagnosis of fascioliasis in experimentally infected cattle by Dot-ELISA and showed high sensitivity, these results suggest that 27 kDa glycoprotein is diagnostic marker for fascioliasis in goat. It was interesting to know that newly juvenile tegument identified in the glycosylated moieties, including the F. hepatica cathepsin B3 (FhCB3) and two proteins of the cathepsin L3 (FhCL3). Moreover, Garcia-Campos et al. (2016) used a kind of liquid chromatography and mass spectrometry to confirm this glycosylation of cathepsins. Unusual paucimannosidic Man2GlcNAc2 glycans were found on position N80 in FhCB3, and in position N153 in FhCL3 on. This result is the first result which proved that F. hepatica cathepsins were of N-glycosylated. Thses addresses the importance of these observations for the production of immunological studies and vaccines, in which the native members of cathepsin FhCL1, FhCL2, and FhCL3 were immunodiagnostic candidates for diagnosis of fascioliasis in sheep with 100% sensitivity (Martínez-Sernández et al., 2018), in buffaloes with 85.2% sensitivity and 86.7% specificity (Aftab et al., 2020), and in goat with 70% sensitivity (Domingo et al., 2018). Overall, these efforts pointed to the role of the glycans and its glycoconjugates antigens in the diagnosis of fascioliasis.
Fasciola Spp. exhibit several mammalian-type globotriaosyl-ceramides, glucosyl- and galactosyl-ceramide, along with Forssman antigen (GalNAc- (β1–3) GalNAc- (β1–3/4) Gal (β1–4/3) Gal(β1–4) Glc-ceramide). Furthermore, F. hepatica neutral glycolipids were differentiated by the presence of terminal Gal (1–6) Gal and Gal (1–4) Gal epitopes, which was similar to that of cestodes. The serological cross-reactivity noticed in the diagnosis of these infections may be attributed to the presence of these glycolipids and corresponding antibodies in F. hepatica, as well as parasitic cestodes infected sera (Bossaert et al., 2000; Wuhrer et al., 2004). glycolipids with Galβ1–3Gal moieties are widely expresses by nematodes, while the GlcNAcβ1-HPO3-6Gal-ceramide acidic moieties were specific for Fasciola (Wuhrer et al., 2003).
Platyhelminths glycans driven immune response
Host immune responses upon platyhelminths infection: Long term survival of parasitic platyhelminths in their host due to process of adaptation or complex co-evolution between the parasites and their host. In the caseof these pathogens, they must find a niche appropriate for mat-uring and spread without damaging or killing the host (Motran et al., 2018). Typically, the host response due to platyhelminths infection is recognized by the induction of type 2 responses which suitable for the parasites survival in their host (Maizels et al., 2004). The parasitic platyhelminths infection have two processes to be kept in mind. First, trematodes and cestodes eggs and larvae migrate in the body and thus induce tissue damage. Therefore, an appropriate response to the pathogen may include containment and tissue repair. This repair mechanism must be kept under control to prevent destructive tissue remodeling and fibrosis. Second, the immune response against trematodes and cestodes infection is not merely a type 2 response but includes anti-inflammatory components. The type 2 response is a reflection of the host immune system recognizing the platyhelminths, while the anti-inflammatory component is likely to reflect the parasite’s adaptation to evade the immune system (MacDonald et al., 2002; Diaz and Allen, 2007). Eosinophils, basophils, and mast cells are activated, IgE is produced, and Th2 cells that secrete IL-4, IL-5, IL-9, IL-10, and IL-13 proliferate in type 2 responses (Coakley et al., 2016). The adaptive immune responses are characterized by B- and T-cells activation. When T- and B-cells recognize the antigen, for which they are specific, they start to clonally expand and induce immunological memory for these antigens. However, there are two drawbacks in this response first; the system isn’t able to recognize the antigen by itself to initiate the response. Second, the response needs too long time about 4-7 days to start to be differentiated into effector cells. Fortunately, the adaptive immune system works side by side with the faster, less fine-tuned innate immune system (Motran et al., 2018). The innate immune response is caused by interaction of innate immune cells such as Antigen-presenting cells (APCs) including, macrophage Ф (M Ф), and dendritic cells (DCs) which by the way these cells have the ability to coordinate the immune effector mechanisms.
The recent advances in the knowledge of platyhelminths glycans in recognition by innate immune cells and sequence of activation of adaptive immune cells against them will be discussed here.
The role of platyhelminths derived glycans in an innate immune response
Parasitic platyhelminths produce diverse range of carbohydrate structures, include many of the well-defined structures that are generally known to be antigenic, the structure of glycan platyhelminths may be host like glycan “self” or non-host like glycan “forigen” (Nyame et al., 2004). It was observed that worms produce N- and O-glycans their structures are similar to those contained in mammalian host “self glycan determinants” for example, Lewis X; LeX; [Gal1,4 (Fucα1-3)GlcNAc], LDN, LDNF, truncated O-glycans, T antigen, (Van Die and Cummings, 2010a). The expression of host-like glycan determinants is remarkable and suggests that platyhelminths may gain advantages by synthesizing such glycans (Van Die and Cummings, 2010b). The expression of “host like” glycans by parasites previously led to the idea of “molecular mimicry,” in which molecules are either derived from the pathogen or acquired from the host to evade recognition by the host immune system. Current discoveries were focused on the ability of parasitic “host like” glycan to binding host glycan-binding proteins (GBPs) and lectin receptor of DCs which result in shaping innate and adaptive immune responses upon infection to promote their survival in their hosts this phenomenon is called “glycan gimmickry,” (Van Die and Cummings, 2010b). Glycan of platyhelminths is commonly terminated with GalNAcb1-4GlcNAc, LDN (Nyame et al., 2004) which is not common in invertebrate host glycans (Van den Eijnden et al., 1995).
The APCs including DCs and MФ initially encounter invading pathogens and are crucial for the regulation of adaptive immune response (Kapsenberg, 2003; Mosser and Zhang, 2008). DCs receptors play a role in their modulation by recognizing specific glycan motifs. DCs receptors including C-type Lectin receptor (CLRs), classes of Toll-like (TLRs) as well as other lectin receptors (Diebold, 2009; Terrazas et al., 2010a). When CLRs recognize glycans, it is thought that they initiate an intracellular signalling cascade that inhibits TLR-mediated signalling (Geijtenbeek and Gringhuis, 2009). Other important receptors are lectin receptors which include macrophage galactose-type C-type lectin (MGL, CD301), and MBL (mannose-binding lectin) such receptors play role in recognizing specific host-like or host non like glycan antigens. The MGL, CD301 receptor recognizes common platyhelminths “host like” glycans containing terminal β- and α-linked GalNAc residues, such as the LDN and Tn antigens (Van Vliet et al., 2005). The DC-SIGN (dendritic cell-specific ICAM3-grabbing non-integrin), MGL (macrophages galactose-type lectin) is another receptors also interact with LDNF and Lewis-X are found in extracts of several platyhelminth (Okano et al., 2001; Geijtenbeek and Gringhuis, 2009). Thus, upon recognition of platyhelminths glycans by DC receptors, controlling the immune response directly or by cross-talk with TLRs will occur. Maturation of DCs results in modulation of host immune system by releasing a panel of cytokines, along with induction of Th2 and T regulatory (Treg) with inhibition of both Th1 and Th17 (Medzhitov, 2007). This modulation in host immune response help parasite to create a safe environment and limiting the attack of the immune system
The role of platyhelminths derived glycans in an adaptive immune response
The study of adaptive immunity to eukaryotic pathogens has historically depended on proteins, rather than glycan antigens. The glycan antigens produced by a parasite during its life cycle are thought to be crucial in escaping detection and clearing from the host, eventually leading to chronic infection (Cruz-Rivera et al., 2019). Many platyhelminths glycans and their glycoconjugates can subvert host immune responses toward Th2 response (Figure 1) by three pathways:
In Th2 responses IL-4, IL-5, IL-9 and IL-13 production were increased (Maizels and Hewitson, 2012). Moreover, an increase in mast cell, eosinophilia and IgE (Homann et al., 2017). The adaptive immune response against many platyhelminths parasites was directed toward glycan determinants which secreted in execratory secretory products of parasites or on the cell surface of parasites. The single glycoconjugates also contain multiple epitopes of glycans. Therefore, anti-glycan antibodies (αGAbs) which represent a “smokescreen” where Antibodies to a specific protein can provide a higher level of bound antibodies and encourage more quantitative complementary fixation or opsonization (Nyame et al., 2004; van Die and Cummings, 2006). The αGAbs (IgA, IgM, IgE and IgG subclasses) are a common feature of trematodes and cestodes infecting sheep, camels, cattle, and pigs (Gómez-García et al., 2006; Sefiddashti et al., 2017).
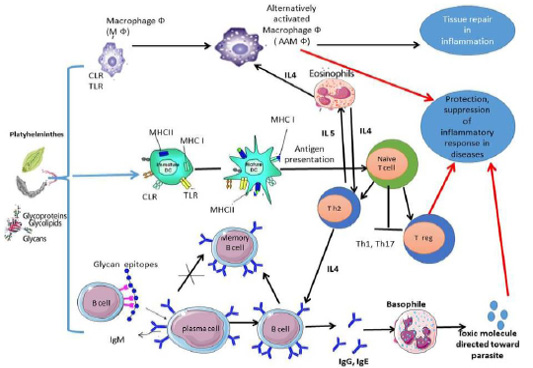
Figure 1: Anti-inflammatory and regulatory immune response was elicited by platyhelminths glycans and glycoconjugates.
Immune response due to platyhelminths glycans
Cestodes: In cestodes, exposure of DC to glycan’s of Taenia spp. inhibite the secretion of IL-15, IL-12, and TNF-α, and inhibit migration of DC (Montero-Barrera et al., 2015). The receptors MGL1 play essential role in innate immunity activation, and antigen recognition also, resistance to infection with T. crassiceps. Deficiency in MGL1 receptors in animals results in increased susceptibility to cysticercosis infection (Montero-Barrera et al., 2015), to indicate that MGL1-recognized glycans are important for parasites to survive in their host. Furthermore, T. crassiceps excreted/secreted glycans play a role in DC maturation by causing naive CD4+ T cells to differentiate into Th2 cells (Terrazas et al., 2010b). These glycosylated antigens also stimulate IL-4 and IL-5, as well as the classic activation of IgG1 and IgE switching by these cytokines (Gómez-García et al., 2006). Dissanayake et al. (2004) proved that T. crassiceps glycan recognized by peritoneal macrophages via TLRs and result in early production of IL-6. Indeed, the Lewis X pentasaccharide of T. crassiceps glycan modulates host responses in a Th-1 direction interferon gamma (IFN-ϓ) and macrophage TLRs (Dissanayake and Shahin, 2007). The D-glucose, D-manose, D-galactose, and D- GalNAc residues of T. solium oncosphere proved its role in antigenicity (Arana et al., 2013). Moreover, the excreted/secreted glycoconjugate antigens of T. saginata cysticerci stimulate a polyclonal B-cell activation to produce IgG and IgM in cattle (Van Kerckhoven et al., 1998). Also, experimentally infected goats with T. multiceps anti-glycan IgG antibody was detected in goat sera from 2 to 17 weeks post-infection, this suggests that the glycan of T. multiceps is a suitable for early diagnosis and vaccination for coenurus infection in goats (Huang et al., 2016).
Interestingly to know that other research groups working on E. granulosus, E. multilocularis have found many similarities with Taenia spp. in the responses of DC following exposure to glycoproteins derived from E. granulosus (Rigano et al., 2007). The glycans antigens of E. multilocularis and glycans antigens of E. granulosus were verified their ability in significant induction of humoral immune responses and play role in parasite evasion from host immune system (Sefiddashti et al., 2017, 2018). The glycoconjugates of E. granulosus protoscolex activate peritoneal B cells differentiation of it in a T-independent manner into IgM-, IgG2b and IgG3 secreting cells, followed by an increase in the secretion of IL-6, IL-10 and TNF-α (Mourglia‐Ettlin et al., 2011). Also, anti-glycoconjugates IgG against E. granulosus was detected in camel (Kamel et al., 2006; El-Shanawany et al., 2019), and in sheep (Khoo et al., 1997). Also, laminated layer Gal-rich mucins of E. multilocularis Em2 (G11)-have immunomodulatory effects that help parasites hosts’ survival. As, the response of IgG against Em2(G11) was elicited independently of CD4+ T cells and in the absence of interactions between CD40 and CD40 ligand (Dai et al., 2001). Also, mixed Th1/Th2-type response due to glycan moieties at the late stage of infection was observed (Gottstein and Hemphill, 2008). The antibodies against αGal(1→4)Gal was strongly detected during infection with CE (Dematteis et al., 2001). Furthermore, glycans of E. granulosus was presented by mature DCs and stimulate the production of IL-4, which suggests that E. granulosus elicit immune response toward Th2 responses (Rigano et al., 2007).
Trematode: Currently, there is little information related to CLR-targeting by Fasciola tegmental glycan, it is likely because there are a wide range of glycan motifs analogous to other trematodes and are play role in the immune regulatory properties of the fluke as host recognize glycans of parasite (Ravidà et al., 2016). In Fasciola infection, its phosphorylated oligosaccharides glycan in the tegument might effect on maturation and function of DC, and lead to Th2/Treg bias (Hamilton et al., 2009). Moreover, Vukman et al. (2013) proved that F. hepatica tegument glycan activates DCs and mast cells to hyporesponsive TLR activation. Indeed, it has been showed that Fasciola oligomannose bind to CLRs leading to increase in the production of IL-10 and IL-4 (Rodriguez et al., 2015). Also, Rodríguez et al. (2017b) proved that Tn antigen (GalNAc-Ser/Thr) component of Fasciola parasite modulates host immune response toward Th2/Treg by interacting with MGL receptor of DCs. As Fasciola are highly glycosylated parasite that has many glycan antigens differ from their mammalian host, it is unsurprising that antibodies against Faisciola protein- and lipid-linked glycans were detected to the host. The anti-glycoconjugate IgG against fascioliasis was observed in naturally infected Egyptian buffaloes (Abdel-Rahman et al., 2016), in experimentally infected goat (Ghosh et al., 2005 a, b).
Glycans of platyhelminths as immunoregulatory therapies
Overall, all of the previous studies also emphasised the importance of increased knowledge about the role of platyhelminths parasitic glycans and their glycoconjugates forms in the recognition and decoding of its molecular patterns, which are important to the development of immunoregulatory responses. These immunomodulatory effects are suggested that platyhelminths glycans can be considered as vaccine and diagnostics candidate against theses parasitic infection because they are essential for parasite survival within the host (Hütter and Lepenies, 2015). Also, currently, they used as therapy for inflammatory and autoimmune diseases (Kuijk and van Die, 2010; Khan and Fallon, 2013).
Last century, the incidence of autoimmune and inflammatory disorders in developed countries has increased exponentially (Farrokhyar et al., 2001; Moroni et al., 2012). It is notable that there is a strong association observed between better sanitation and a sharp increase in autoimmune, and inflammatory disorders (Elliott and Weinstock, 2012; Wiria et al., 2012). In the late 1980s the Hygiene Hypothesis was originally formulated, current thought in the field has been modified and is currently known as the Old Friends Hypothesis and/or Biome Depletion Theory (Parker and Ollerten, 2013). The significant and sustained decline in pathogenic and parasite infection in developed countries is currently assumed to affect the immune system’s immunoregulatory mechanisms (Rook, 2012). Particularly, the exposure to parasitic helminths (platyhelminths and nematodes) has been shown to correlate with atopic, autoimmune, and autoinflammatory diseases protection. More than 40 years ago, the first study on treatment by helminths focused on allergic diseases, such as asthma in humans and animal models, this finding indicating the potential benefits of helminths for health (Turton, 1976). Currently, however, several studies have indicated that helminth infections or use of helminth antigens, also able to decrease inflammation which associated with autoimmune diseases (Sobotková et al., 2019). Although the immunology caused by worms is resolved, almost no studies attract attention to the helminth molecules responsible for these defensive effects, as well as the molecular mechanisms that underpin them; one of these molecules is helminth-derived glycans (Kuijk and van Die, 2010).
The imbalance in immunity when the normal body molecules are pathologically attacked by the immune system that can lead to (1) Autoimmunity, which is triggered by Th1 and Th17 cells with macrophages and DCs associated with an increase in pro-inflammatory cytokines. (2) Allergic, Th2 cells predominate in allergic people, with fewer Th1 cells and a drop in Treg and regulatory cytokines including IL-10. The secretion of IL-5, IL-4, and IL-13 results in elevation in level of an allergen-specific IgE, eosinophilia, mast cell, and basophil (Mangan et al., 2006). Platyhelminths express a wide range of glycans on their surface and execratory secretory products as mentioned before. Some of these glycans are especially interesting as they regulate host immune responses by their interaction with C‐type lectins on Dcs and therefore result in T‐cell polarization (Kuijk and van Die, 2010). So, these platyhelminths glycans and glycoconjugates moieties may benefit as a therapeutic weapon against autoimmune diseases and inflammatory disorders, because they create a balance between autoimmune Th1/Th17 and allergic Th2 responses.
CONCLUSIONS AND RECOMMENDATIONS
ACKNOWLEDGEMENTS
The manuscript has never been published in another journal. The authors have agreed to the terms specified in the copyright assignment form.
Novelty Statement
The novelty of the presented review was largely depend on in-depth knowledge of platyhelminths glycan structures and their roles in diagnosis and immune response in farm animals.
Author’s Contribution
The review article idea, literature search, data analysis, and revised the work by Eman E. El Shanawany.
Conflict of interest
The authors have declared no conflict of interest.
References





