Advances in Animal and Veterinary Sciences
Research Article
Anti-Inflammatory Effects of Evening Primrose and Extra Virgin Olive Oil on Arthritc Rats
Basant M. Mahmoud1, Sana’a O. Ebrahim1*, Mohamed A. Kandeil2
1Biochemistry Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt; 2Biochemistry Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt.
Abstract | Rheumatoid arthritis is characterized by amplification of pro-inflammatory cytokines, white blood cells, and platelets contributing to acute edema and hyperalgesia. The aim of this study is to evaluate the enhancing influence of extra virgin olive oil (EVOO) and Evening primrose oil (EPO) in adjuvant-induced arthritic rat models and compare between EPO and EVOO effects. A subcutaneous injection of 200 µl Freund’s complete adjuvant into footpad of the right hind leg of male Wistar rats at two consecutive days to induce Rheumatoid arthritis. Arthritic rats were treated orally with Extra Virgin olive oil and Evening Primrose Oil at doses of 5 mg/kg. b. wt./day for 10 and 21 days and then compared to corresponding arthritic control at the same periods. Blood sample collection, scarification of rats, and preparation of ankles and spleen for histopathological investigations were carried out at the end of each treatment period. Statistical analysis was carried out using Graph Pad Instat software (version 8, ISS-Rome, Italy). The results showed edema at the right hind limb a few hours after CFA injection. EVOO and EPO treatments ameliorated the elevated ankle diameter, white blood cells count and platelets number in arthritic rats. The Treatments decreased mitotic figures and lymphoblasts activation in spleen specimens. Enhanced inflammatory biomarkers (rheumatoid factor, tumor necrosis factor-alpha, interleukin-1beta, and interleukin-6) levels in arthritic rats were markedly decreased due to EVOO and EPO treatments administration. Conclusion: EVOO and EPO had significant anti-inflammatory effects on arthritic rats. EVOO and EPO treatments lead to a significant improvement of blood cell count, histological changes, and augmented inflammation markers. More studies on EVOO and EPO combination with other Disease-modifying antirheumatic medications are recommended to increase their effectiveness with less toxicity.
Keywords | Rheumatoid arthritis, Cytokines, Autoimmunity, Interleukins, Extra virgin olive oil (EVOO), Evening primrose oil (EPO)
Received | May 24, 2021; Accepted | June 17, 2021; Published | August 25, 2021
*Correspondence | Sana’a Omar Ebrahim, Biochemistry Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt; Email: Ls.sanaa.omar@gmail.com
Citation | Mahmoud BM, Ebrahim SO, Kandeil MA (2021). Anti-inflammatory effects of evening primrose and extra virgin olive oil on arthritc rats. Adv. Anim. Vet. Sci. 9(10): 1684-1691.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1684.1691
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Lukkananukool et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune illness with a percentage of 1% of the population. It affects women more than men (Narendhirakannan et al., 2007). The development of RA could be enhanced by some genetic and environmental risk factors with a 50% risk (Smolen et al., 2018). Freund’s complete adjuvant (FCA) mirrors the pathology of RA with hyperplasia of the synovial tissues, joints inflammation, and demolition of the cartilages and bones in the affected area (Saratha and Subramanian, 2012). Evening primrose oil (EPO) is extorted from Oenothera Biennis seeds. EPO was evaluated medically for its therapeutic influence to treat different diseases in controlled clinical trials like rheumatoid arthritis, various cancers, breast cysts, and schizophrenia (Bayles and Usatine, 2009). Nutritional omega-3 and omega-6 fatty acids can oppose the bad effects of unsaturated fatty acids and eicosanoid actions in tissues, which cause various inflammatory and immunologic pathogenesis. Several studies reported the useful influence of EVOO polyphenols in several inflammatory diseases, including RA (Impellizzeri et al., 2012). EVOO beneficial effects have been attributed to its high oleic acid contents, also there is increasing evidence supports the useful effects of EVOO intake for its minor highly bioactive components including phenolic compounds, sterols, vitamins (α- and γ-tocopherols), triterpene alcohols, β-carotene, hydrocarbons (squalene), and phytosterols (Cárdeno A et al., 2013).
Our study aimed to assess the advantageous effects of primrose oil and olive oil as an anti-inflammatory remedy in rheumatoid arthritis and compare them.
MATERIALS AND METHODS
Experimental animals
The protocol of the current experiment was approved with an Ethics approval number BSU-IACUC 021-0149 (Institutional Animal Care and Use Committee Beni-Suef University) and All animal procedures are in accordance with their guidelines in the Biochemistry department, Faculty of science for 1 Month includes Housing and treatments procedures.
Male Wistar rats weighting from110 to 130 g were housed in plastic cages with good aeration at normal dark/light cycle of 12 h, humidity (55 ± 5%), and temperature (25 ± 5 °C) and were provided with water and a standard balanced diet via ad libitum. We bought them from the National Research Centre, Egypt, and rats were then isolated under observation for 2 weeks in separate isolation units.
Animals with weight over 140 g, less than 120 g or who suffered from any illness were excluded from the experiment. Animal’s we redistributed after their adaptation time into separate cages, each with 6 rats and marked with treatment type and time. At the end of each period, rats were anesthetized by inhalation using light diethyl ether (5%); ankle circumstances were measured and recorded. Blood samples were drawn from the middle canthus of the eye.
Animals were divided into eight groups of 6 rats and classified:
At 10 days Post inoculation: The first group (G1): normal rats were regarded as a control group and weren’t suffering from any illness affecting measured parameters. The second group (G2): rheumatoid arthritis untreated rats. The third group (G3): EPO-treated rats. The fourth group (G4): EVOO-treated rats.
At 21 dpi: The fifth group (G5): normal rats were regarded as a control group and weren’t suffering from any illness affecting measured parameters. The sixth group (G6): RA untreated rats. The seventh group (G7): EPO-treated rats. The eighth group (G8): EVOO-treated rats.
Creation of rheumatoid arthritis
Approximately 0.2 ml of Freund’s complete adjuvant mainly contains a suspension of heat-killed Mycobacterium tuberculosis in mineral oil was infuse into rats’ right hind footpad at two consecutive days as previously described by (Impellizzeri et al., 2011). FCA was purchased from Sigma Company, Cairo, Egypt.
Treatment protocol
Evening primrose oil (EPO)
Evening Primrose oil was purchased from EVA pharm co. (Cairo, Egypt) as a gelatin capsule containing EPO 1000 mg. 5 mg/kg. b. wt./day was given orally daily at 9:00 am as previously described (Impellizzeri et al., 2011). The treatments were given using oral gavage needle gauge 18 2-3 cmm length and ball diameter.
Extra virgin olive oil (EVOO)
An EVOO bottle was purchased from a traditional market in Beni-Suef, Egypt. It was of Illiada product made in Italy. About 5 mg/kg. wt./day was given orally daily at 9:00 am as previously described by (Impellizzeri et al., 2012). The treatments were given using oral gavage needle gauge 18.
Whole blood and serum preparation
Blood samples were drawn in standard blood vacutainer tubes (Gold and Purple), then centrifuged at 1435 x g for 15 minutes and non-hemolyzed sera were pipetted and divided into three Eppendorf for each animal and stored at -20°C till utilization.
Detection of total leukocytes count, platelets, and serum cytokines
After shaking EDTA blood (Purple tubes), PLT and TLC tests were carried out using Turk’s fluid kits purchased from Egyptian Diagnostic Media Company, Egypt according to factory directives. Serum RF levels (IU/ml) tested according to Halbert et al. (1980) method using Elisa kit (RF) obtained from CUSABIO Company, USA code CSB-E13666r. TNF-a, IL-1b, and IL-6 levels were determined by using specific ELISA kits (Catalog no. PMTA00B, PMTA00B, PM6000B) purchased from R and D systems company, USA.
Histopathological investigations
Under anesthesia by inhalation light diethyl ether, the ankle perimeter of the right hind legs was measured and recorded. Spleen and hind right leg ankle with articular bones were removed then washed in saline and finally fixed in 10% neutral buffered formalin (Alers et al., 1999). In the Microanalytical Center, Faculty of Science, Beni-Suef University; fixed ankles were decalcified with 10% ethylene diamine tetra-acetic acid (EDTA) then decalcified ankles and spleen tissues were consistently processed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin according to the method of Bancroft et al. (1996). The Microscope magnification obtained by multiplying the original magnification by 100.
Statistical analysis
Graph Pad Instat software (version 8, ISS-Rome, Italy) was used for Statistical analysis. Groups of data were compared with an unpaired t-test one-way analysis of variance (ANOVA) followed by two-way ANOVA, Values of P <0.05 were considered as significant. Data were expressed in tables and figures as mean ±SEM.
RESULTS AND DISCUSSION
Ankle edema diameter and blood cells count (platelets and white blood cells)
Arthritic rats showed significant enlargement in ankle sizes at 10 and 21 days, and most potent increases were recorded at 10 days post-CFA injection. EPO and EVOO treatments significantly decreased the enlargement values recorded at 10 and 21 days. EPO showed a greater positive effect of 10 dpi than EVOO but at 21 days there was not a significant difference.
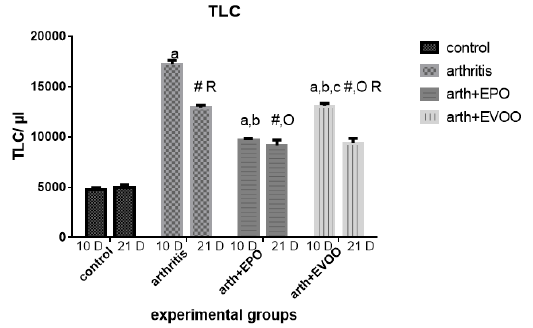
White blood cells were raised in arthritic groups as compared with normal groups in study periods (Table 1 and Figure 2). The eminent effects were recorded at 10 dpi. EPO and EVOO treatments cause a significant improvement at 10 and 21 dpi but EPO decreased the count than EVOO at 10 and 21 days. EVOO showed better declines at 21 days compared to 10 days treatment with no significant difference in EPO between 21- and 10-days treatment groups.
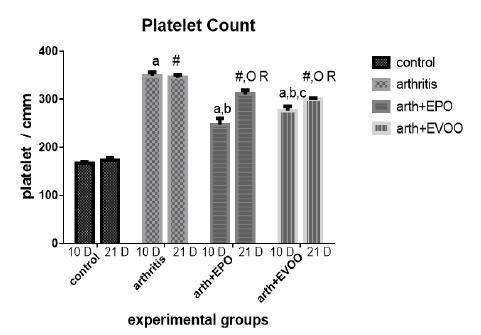
Platelet’s enumeration (Table 1 and Figure 3) accretion was observed at 10 and 21 days in arthritic groups as compared with normal groups, but at 10 dpi was squeakier. EPO and EVOO minify them in the study periods. EPO decreased the count more than EVOO at 10 and 21days. Oils treatments for 21 days had better effects than for 10 days.
Serum inflammatory biomarkers and interleukins results
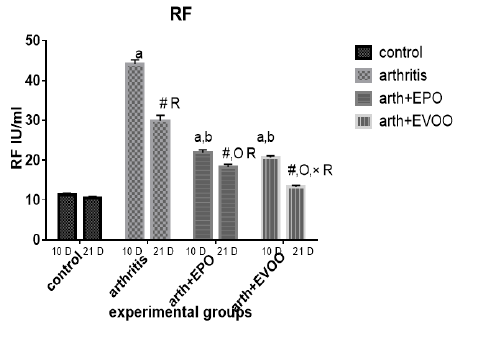
Serum RF was significantly increased in untreated rats at 10 and 21 days compared to normal rats but the highest level was recorded at 10 days. EVOO and EPO treatment decreased these magnified levels at 10 and 21 days. EVOO treated groups showed a better effect compared to the EPO group at 21 days (Table 2 and Figure 4).
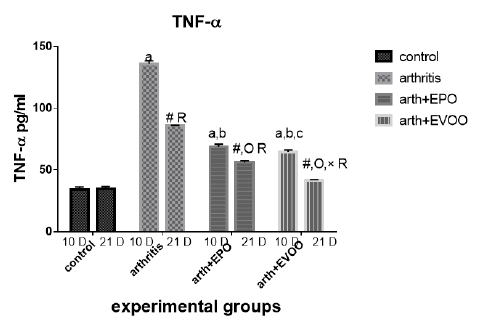
Serum TNF-α (Table 2 and Figure 5) was significantly increased in untreated rats at 10 and 21 days compared to normal rats but the highest level at 10 days. EVOO and EPO treatment decreased the elevated levels at 10 and 21 days. EVOO treated groups showed a better effect compared to the EPO group at 10 and 21 days.
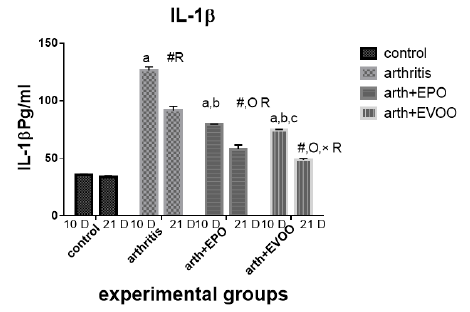
Serum IL-1β (Table 2 and Figure 6) was significantly increased in untreated rats at 10 and 21 days compared to normal rats but the highest level at 10 days. EVOO and EPO treatment decreased these levels at 10 and 21 days. EVOO treated groups showed a better effect compared to the EPO group at 10 and 21 days.
Table 1: Edema diameter, TLC and platelets count in our study groups.
| Platelets (cells/cmm) | TLC (cells/ul) | Edema (cm) | Parameter Rats’ groups |
| 166.3±3.73 | 4717±263.8 | 2.0±0.0 | Normal 10dpi |
| 348.3±7.74 a | 17242±374.7 a | 3.07±0.021 a | Arthritis 10 dpi |
| 247±13.25 a,b | 9167±543.2 a,b | 2.47±0.021 a,b | EPO 10 dpi |
| 276.3±9.0 a,b,c | 13.067±266.7 a ,b, c | 2.55±0.043 a,b,c | EVOO 10 dpi |
| 172.8±5.32 | 5000±262.0 | 2.1±0.068 | Normal 21dpi |
| 345±5.44 # | 12917±238.6 # R | 2.733±0.033 #R | Arthritis 21 dpi |
| 310.5±7.9 #O R | 9697±151.9 # O | 2.42±0.048 #O | EPO 21 dpi |
| 299.3±3.64 #O R | 9383±470 # O R | 2.48±0.48 #OR | EVOO 21 dpi |
Data expressed as MEAN ± SE. number of rats is 6 for each group. Values of P <0.001 were regarded as significant the differences between groups are expressed as follow: a significantly different from the control 10 dpi gp. # Significantly different from the control 21 dpi gp; b significantly different from arthritis 10 dpi gp. O significantly different from the arthritis 21dpi gp; C significantly different from the EPO 10 dpi gp. × significantly different from the EPO 21 dpi gp; R significantly different from its corresponding 10 dpi gp.
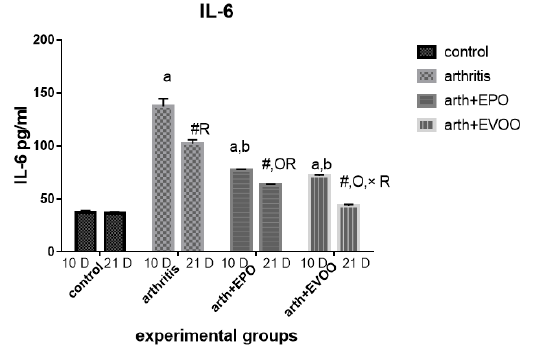
Table 2: Serum inflammatory biomarkers in arthritis, EPO and EVOO treated rats.
| IL-6(Pg/ml) | IL-1(Pg/ml) | TNF-α(Pg/ml) | RF (IU/ml) | Parameter rats groups |
| 37.53±1.67 | 35.43±1.0 | 33.83±2.27 | 11.23±0.50 | Normal 10dpi |
| 137.2±7.39 a | 126.2±3.0 a | 135.9±2.47 a | 44.12±1.1 a | Arthritis 10 days |
| 77.23±0.73 a,b | 79.15±0.36 a,b | 68.77±2.12 a,b | 21.9±0.73 a,b | EPO 10 dpi |
| 71.8±0.81 a,b | 74.43±0.61 a,b,c | 64.63±1.52 a,b,c | 20.7±0.41 a,b | EVOO 10 dpi |
| 36.52±1.46 | 33.87±1.1 | 34.83±1.64 | 10.48±0.50 | Normal 21dpi |
| 102±3.63 # R | 91.33±3.6 # R | 85.52±0.53 # R | 29.87±1.46 # R | Arthritis 21 dpi |
| 63.2±0.66 #O R | 57.78±3.78 #O R | 56.07±1.44 #O R | 18.27±0.73 # OR | EPO 21 dpi |
| 43.63±1.11 #O× R | 48.55±1.17 #O× R | 41.1±0.64 #O× R | 13.32±0.34#O× R | EVOO 21 dpi |
Data expressed as MEAN ± SE. number of rats is 6 for each group. Values of P <0.001 were regarded as significant the differences between groups are expressed as follow: a significantly different from the control 10 dpi gp. # Significantly different from the control 21 dpi gp; b significantly different from arthritis 10 dpi gp. O significantly different from the arthritis 21dpi gp; C significantly different from the EPO 10 dpi gp. × significantly different from the EPO 21 dpi gp; R significantly different from its corresponding 10 dpi gp.
Serum IL-6 (Table 2 and Figure 7) was significantly increased in untreated rats at 10 and 21 days compared to normal rats but the highest level at 10 days. EVOO and EPO treatment decreased the recorded levels at 10 and 21 days. EVOO treated groups showed a better effect compared to the EPO group at 21 days.
The histological changes of ankle diameter and spleen
As showed in Figures 8 and 9, microscopic assessment of normal rats’ ankles showed normal structures while untreated rats show evidence of focal necrosis in articular cartilage and inflammatory cells infiltration in 10 dpi and 21 dpi which decreased in EVOO and EPO (10 and 21 dpi) treated groups.
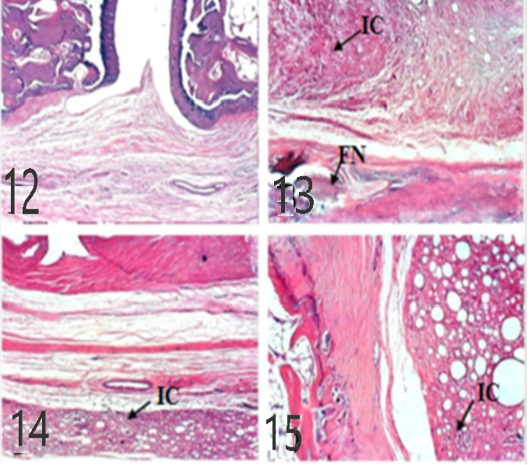
Spleen histological changes were illustrated in Figures 10 and 11. We reported No histopathological changes in the Normal rats. the untreated arthritic rats showed lymphoblasts proliferation and mitotic figure after 10 and 21 days of RA induction. Spleen slides showed inflammatory cells infiltration in EPO and EVOO treated groups for 10 days while in 21 days treated groups spleen was recovered and no histopathological changes were founded.
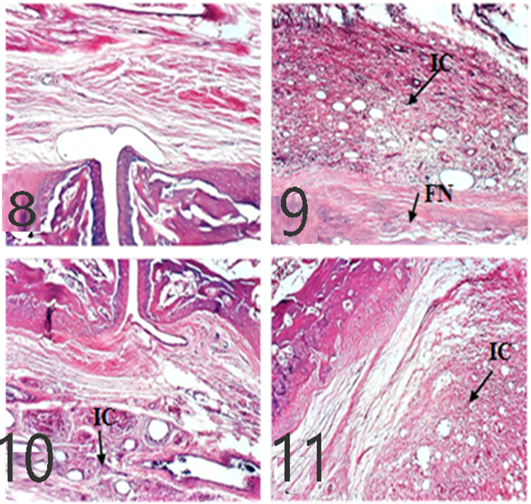
Figure 8: Ankles of rats 10 dpi by (H and E x 100). A: No histopathological changes in the control (normal) group; B: inflammatory cells (IC) infiltration and focal necrosis (FN) of articular cartilage in arthritis group; C: Few inflammatory cell infiltrations in the EPO-treated group; D: Few inflammatory cell infiltrations in EVOO-treated group.
Figures 9: Ankles of rat 21 dpi showing: by (H and E x 100). A: No histopathological changes in the control (normal) group; B: inflammatory cells (IC) infiltration and focal necrosis (FN) of articular cartilage in arthritis group; C: Few inflammatory cell infiltrations (H and E) in the EPO-treated group; D: Few inflammatory cell infiltrations (H and E) in EVOO-treated group.
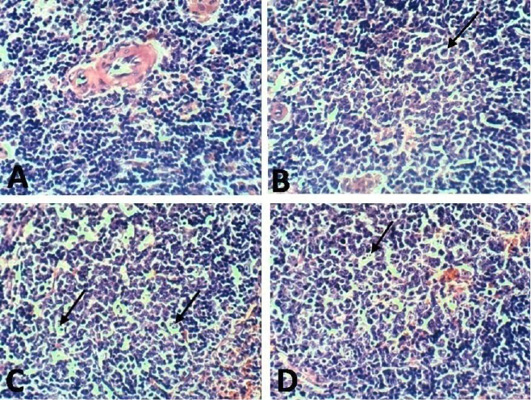
Figure 10: Spleen of rat 10 dpi showing by (H and E x 100). A: No histopathological changes in the control (normal) group; B: lymphoblasts proliferation and mitotic figure in arthritis group; C: inflammatory cells infiltration in EPO treated group; D: inflammatory cells infiltration in EVOO treated group.
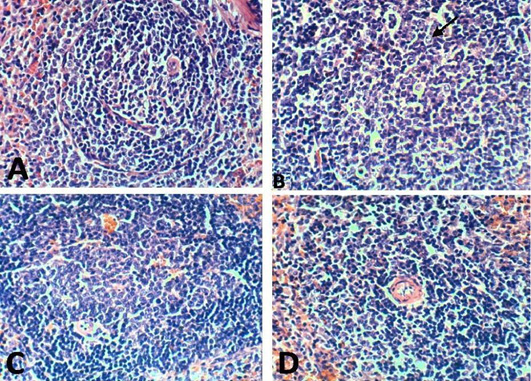
Figure 11: Spleen of rat 21 dpi by (H and E x 100) showing. A: No histopathological changes in the control (normal) group; B: lymphoblasts proliferation and mitotic figure in arthritis group; C: No histopathological changes in the EPO-treated group; D: No histopathological changes in the EVOO-treated group.
Rheumatoid arthritis (RA) is a morbidness due to autoimmunity errors that causes’ inflammatory cells attack and pathological changes in the lining of the joints (Széles et al., 2007).
Since pathogenesis of adjuvant-induced arthritis in rats resembles human arthritis, arthritis in rats is the most suitable technique to study the anti-arthritic and anti-inflammatory gadget of olive oil and primrose oil.
The current study revealed a significant increase in leg ankle diameter in CFA-induced rats at 10 and 21 days compared to normal rats but the highest level at 10 days this was the same as (Ahmed et al., 2017) results.
Edema, inflammation, thickening of the synovial membrane, redness, and congestion in blood vessels that can be observed by both morphological and histological inspections are due to biphasic inflammatory response termed early (acute) and late (chronic) phases (Taniguchi et al., 2004). Edema may be attributed to amplified IL-1ß levels which also causes hyperalgesia (Billiau and Matthys, 2011).
Histological slides observation showed that EVOO could decrease the inflammatory cells infiltrated in the ankle’s joints after 10- and 21-days treatments. These results were similar with a study conducted by Terzuoli et al. (2010). When treating rats orally with EPO for 10 and 21 days and observed its enhancing effect, El-Sayed (2014) argued that effect was related to gamma-linolenic acid which represents about 9% contained in EPO.
In this study, the arthritis inflammation observed with significant higher white blood cells and thrombocyte count, these outcomes are parallel with Kumar et al. (2004) findings that; the increase in the cell number are due to their participation in systemic inflammatory response in the body. Interleukins especially those entangled in cell-mediated immune reactions, activate and maturate WBCs and platelets supporting the immune system fight against invading pathogenic microorganisms (Vander Borght et al., 2001; Patel and Pundarikakshudu, 2015). Our results illustrated that EVOO decreased the cells count compared to untreated groups; this was also illustrated by Mokhtari et al. (2019) and Singh et al. (2012) who stated that olive oil polyphenols decreased WBCs due to its anti-oxidative, anti-apoptotic, and anti-inflammatory specifications. EVOO oleic acid decreases platelet count, fibrinogen, monocytes sticking, and factor VII which inhibits the blood clots formation (Bogani et al., 2007). Another EVOO constituent is the Phenols which reduce TXB2 and oxidative stress harm in both humans and experimental animal models. Our study results illustrated that EPO decreased the cells count compared to the untreated groups; as it was also illustrated by Riaz et al. (2009) that showed EPO treatment decreased platelet count.
IL-1β, TNF-α, and IL-6 significantly higher in untreated arthritic rats in 10 and 21 dpi, they might be released by activated macrophages in the inflammation cascade (Jazayeri et al., 2010). RA inflammation pathogenesis has been attributed in sundry studies to the activation of macrophages, CD4+ T cells, auto-antibodies, B cells, neutrophils, free radicals into the synovial stroma which led to an excess output of IL-1β, TNF-β, IL-6, and cytokines and increased oxidative stress characterized by decreased antioxidants and increased lipid peroxidation (Tag et al., 2014).
Our results showed that EVOO treatment decreased TNF-α, IL-1β, and IL-6 levels in 10 and 21 days. These results were founded by Janahmadi et al. (2015) and Miles et al. (2005) accentuated thatIL-1β and TNF-α lowered due to oleuropein treatment (20 and 30 mg/kg) which diminished osteoblasts RANKL expression to hamper osteoclast differentiation and maturation (Serra et al., 2017), authors attributed this effect due to olive oil ώ-3 FAs which restrain protein kinase C from tissues (as spleen and brain), macrophages, and lymphocytes so preventing NF-κβ activation (Alwi et al., 2007) also olive oil phenol (Hydroxytyrosol) which decreased the production of TNF (Zhang et al., 2009) by decreasing ROS, iNOS and COX-2 expression (Serra et al., 2017). Minor compounds of EVOO such as maslinic acid (pentacyclic triterpenoid), Erythrodiol (triterpenes alcohols), and oleanolic acid (triterpenes acid) have shown anti-inflammatory activities reported in-vitro studies (Navarini et al., 2017).
EPO treatment showed a significant ameliorating effect on TNF-α, IL-1, and IL-6 levels days in arthritis rats. These results were in parallel with a study results of Abo-Greshaet al. (2014). IL-6 and TNF-α decreased due to EPO and statins were due to activation of peroxisome proliferator-activated receptor-γ and decrease NF-κB (Das UN 2001). GLA and DGLA within the body are converted to anti-inflammatory compounds which suppress the inflammatory cascades. ɤ-linolenic acid existent in EPO (8–10%), converted to DGLA, which is a predecessor of anti-inflammatory eicosanoids so EPO usage could increase prostaglandin E1 (PGE1) and PGE2 in tissues (Wang et al., 2014). In addition, DGLA is a competitive inhibitor with AA for the COX pathways. This inhibition is a major mode of action in the way that dietary sources of DGLA (e.g., EPO) reduce inflammation (Takai et al., 2009).
As showed in our results the IL-1 and TNF-α in EVOO rats treated in 10 and 21 days was the lowest compared to the EPO treated rats while the IL-6 showed the same pattern when treated in 21 days.
CONCLUSIONS AND RECOMMENDATIONS
Our results show that inflammatory cytokines and inflammation process is implicated in rheumatoid arthritis and complementary medicines include extra virgin olive oil and primrose oil could decrease their levels. The current study shows that the EVOO anti-inflammatory effects are better than EPO effects.
ACKNOWLEDGMENTS
This research didn’t receive any specific grant from any funding agencies.
AUTHOR’S CONTRIBUTION
MK and BM have contributed in suggesting design of the study. All authors have conducted research, provided research materials, and wrote the article. All authors critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Competing interests
The author have declared no conflict of interest.
REFERENCES