Advances in Animal and Veterinary Sciences
Research Article
Anti-Inflammatory Effect of Coleus amboinicus Leaves Extract on Uric Acid-Induced Nephrotoxicity Rats
Rondius Solfaine1, Lailatul Muniroh2*, Sadarman3, Apriza4, Agung Irawan5
1Department of Pathology, Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, Jl. Dukuh Kupang XXV/54, Surabaya, 60225, Indonesia; 2Department of Nutrition, Faculty of Public Health, Universitas Airlangga, Kampus C UNAIR, Mulyorejo, Surabaya, 60115, Indonesia; 3Department of Animal Science, Sultan Syarif Kasim State Islamic University, Pekanbaru, Indonesia; 4Nursing Department, Faculty of Health Sciences, Pahlawan Tuanku Tambusai University, Kampar, 28412, Indonesia; 5Vocational Program of Animal Husbandry, Universitas Sebelas Maret, Surakarta 57126, Indonesia.
Abstract | Recently, food consumption has changed following to sedentary lifestyle and high purine diets. This study examined the anti-inflammatory effect of Coleus amboinicus leaves extracts on uric acid-induced nephrotoxicity rats. Twenty-four Wistar rats were distributed into 3 groups (n = 8 rats for each group). The experiment was performed based on the following treatment: P0= rats received normal saline after being fasted for 12 h; P1= rats were induced with 500 mg/kg BW uric acid, 750 mg/kg BW oxonic acid, and 0.1% sodium carboxymethyl cellulose (CMC-Na) (positive control); and P2= induced-rats similar to P1 but received 500 mg/kg BW CA plant extract. The experiment lasted for 14 days. All rats were sacrificed for blood samples and tissue fixation on day 15 and were analyzed for serum creatinine and blood urea nitrogen (BUN), glutathione peroxidase (GPx), and transforming growth factor-β1 (TGF- β1) concentrations. Kidney tissues were collected for immunohistochemical and hematoxylin-eosin staining. Results showed that induction of uric acid (P1 and P2) revealed necrotic lesions in the tubular membrane. Concurrently, serum creatinine and BUN concentration increased (p<0.05) in the P1 group while the P2 can compensate for such increasing trends, giving a similar value to P0 (p>0.05). Treatment with CA leaves extract at 500 mg/kg BW (P2) increased GPx and TGF-β1 concentrations (p<0.05) compared with the positive control group (P1). Immunohistochemical analysis showed an increase in the expression of CD-68 and CD-163 macrophages characterized by brownish aggregate in the area of renal tubules and glomerulus in the positive control and treatment groups compared to the control group. This evidence suggests that Coleus amboinicus leaves extract can be used as an anti-inflammatory agent because it was effective to inhibit acute kidney failure by restore BUN and serum creatinine to normal concentrations.
Keywords | Anti-inflammatory agents, Coleus amboinicus, Uric acid, Toxic chemicals
Received | March 15, 2021; Accepted | June 16, 2021; Published | August 15, 2021
*Correspondence | Lailatul Muniroh, Department of Nutrition, Faculty of Public Health, Universitas Airlangga, Kampus C UNAIR, Mulyorejo, Surabaya, 60115, Indonesia; Email: lailamuniroh@fkm.unair.ac.id
Citation | Solfaine R, Muniroh L, Sadarman, Apriza and Irawan A (2021). Anti-inflammatory effect of Coleus amboinicus leaves extract on uric acid-induced nephrotoxicity rats. Adv. Anim. Vet. Sci. 9(10): 1553-1558.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1553.1558
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Solfaine et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In the last decade, people’s preference for food has changed following the development of lifestyle and technology. Consumption of modern diets which are typically high in fat, protein, and purines contents increases worldwide. High purine intake is known to increase uric acid concentration. Purine derivatives are produced by either metabolism of food nutrients or de novo synthesis from nucleotide precursors by converting hypoxanthine (monoxide) to xanthine (dioxypurine) into uric acid (trioxypurine) as the final product. Uric acid is insoluble in serum and is maintained in low concentrations at normal body temperature (Shimada et al., 2009; Kensara, 2013). In humans, uricase, an enzyme with the ability to oxidize purine molecules, is not produced due to the mutation of the gene encoding uricase. The absence of the uricase resulted in a ten-fold increase in uric acid concentration in serum plasma (So and Thorens, 2010). In addition, the balance of sodium ion exchange greatly affects uric acid excretion and the insoluble nature of uric acid makes it difficult to excrete. The accumulation of allantoin compound as the final uric acid metabolite also a factor in increasing uric acid levels in the human body (Zhou et al., 2014).
The accumulated uric acid concentration can prolong to cause acute renal damage and nephropathy in cases of chronic hyperuricemia incidence. This condition is characterized by the accumulation and blockage of urate crystals in the lumen of renal tubules, as well as tubular epithelial proliferation with inflammatory cell infiltration. Nephropathy is an acute renal failure with glomerulosclerosis lesions, thickening of the glomerular basement membrane, mesangial cell proliferation, decrease in glomerular filtration rate, albuminuria, high blood pressure, and fluid retention (Kim et al., 2000; Mazzali et al., 2001; Balakumar et al., 2008).
Uric acid deposition promotes macrophage infiltration and expression of monocyte chemoattractant protein-1 (MCP-1), increases renin expression and cyclooxygenase-2 (COX-2) enzymes as well as damages kidney tissue in the form of glomerulosclerosis, interstitial fibrosis, and renal arteriosclerosis. Uric crystals can induce an acute inflammation through the activation of the interleukin-1β receptor (TIR), transforming growth factor-1β (TGF-1β), Caspase-NALP3-1, expression of TNF-β at the Toll-like receptor-4, and increase the expression of vascular endothelial cells (ICAM-1 and VCAM-1) (Kang et al., 2002). The transforming growth factor-1β (TGF-1β) is an anti-inflammatory cytokine, autocrine and paracrine to regulate cell proliferation, apoptosis, chemotaxis, the immunity that can respond to the inflammatory and immune processes as well as tissue repair (fibrosis) in the kidney tissue (Tang et al., 2014). Additionally, CD-68 macrophage expression functions as an inflammatory mediator, scavenger of apoptotic cells, produce growth factors and induces proinflammatory cytokines such as IL-6 and TNF-β during the inflammatory process in kidney tissue. Macrophage expression indicates an increase of phagocytosis in inflamed tissues.
Coleus amboinicus (CA) plant is widespread and has been consumed daily for medicinal purposes and used as food supplements by people worldwide. This plant contains many active compounds including monoterpenoids, sesquiterpenoids, diterpenoids and phenolics, 3 methyl 4 isopropyl phenols, squalene, caryophyllene, phytols, alkaloids, glycosides, flavonoids, quinones, tannins, phenols, and terpenoids (Rice et al., 2011; Lukhoba et al., 2006; Soni and Singhai, 2012; Pillai et al., 2011; Patel et al., 2010). These active compounds are known to have an antioxidant effect that can be used as preventive agents for the oxidative process of free radicals by inhibiting, scavenging, and neutralizing the free radicals. The major action of the constituents is attributed to their ability to activate enzymes that act as antioxidants such as superoxide dismutase, catalase, and glutathione peroxidase (GPx) (Krishnaiah et al., 2007). The GPx can bind selenium to convert H2O2 and hydroperoxide into active compounds without any toxic effects on the body. This enzyme works with other enzymes such as SOD, CAT in neutralizing H2O2. By increasing free radicals and the accumulation of lipid peroxide, it increases the activity of GPx in the tissue (Singh et al., 2013). Coleus amboinicus has been reported to have anti-inflammatory properties in rheumatoid arthritis, anticonvulsants, antitumor, and functions as a stimulant for breast milk secretion as well as acts as anti-nephrolithiasis in experimental animals (El-Hawary et al., 2012; Bhatt et al., 2013; Jose et al., 2005; Pallani et al., 2010; Chang et al, 2010; Gurgel et al., 2009). Considering the beneficial effects of the CA plant, this study aimed to examine the anti-inflammatory effect of Coleus amboinicus leaves extracts on uric acid-induced nephrotoxicity rats.
MATERIALS AND METHODS
This research has been approved by the ethical committee of the Animal Care and Use Committee (ACUC), Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia (approval number: 518-KE/2019).
Sample preparation and extraction
Coleus amboinicus plant was purchased from Traditional Flower Market (Bratang Traditional Market) located in Surabaya, Indonesia. The plant has been identified by Botanical Garden Conservation Center, Pasuruan East Java, Indonesia. Fresh Coleus amboinicus plants were separated from the root, stems, and leaves then washed with tap water, and weighed. The clean leaves were cut into small pieces, dried in a normal temperature room (~25° C) without exposure to sunlight to maintain the active compounds of the leaves. The dried leaves were sieved and macerated using 96 % ethanol as a solvent. The Coleus amboinicus leaves extract was dissolved in a suspension containing 0.1% sodium carboxymethyl cellulose (CMC-Na). The CMC-Na suspension was used for treatment in the positive control group for 14 days.
Experimental animals
Twenty-four Wistar rats were divided into 3 groups where each group consisted of 8 animals. The treatment groups were: P0 = control group that received normal saline after being fasted for 12 h; P1 = positive control group that induced with uric acid (500 mg / kg BW), oxonic acid (750 mg/kg BW), treated with sodium carboxymethyl cellulose 0.1 % (CMC-Na 0.1 %). The P2 = treatment group that induced with uric acid (500 mg / kg BW), oxonic acid (750 mg/kg BW) and treated with CA extract (500 mg / kg BW) for 14 days.
Sample collection and analysis
All rats were sacrificed by euthanization for blood serum and kidney tissue collection on day 15. Serum creatinine and blood urea nitrogen (BUN) levels were analyzed by colorimetric method (Diasys diagnostic GmbG, Germany) to evaluate the renal function. The concentration of glutathione peroxidase (GPx) was determined by rat glutathione peroxidase total assay (Biostar, USA) while the transforming growth factor-β1 (TGF- β1) was determined by using the Enzyme-linked immunosorbent assay kit (R and D, USA). Kidney tissue was fixed in 10% buffered neutral formalin (BNF) and stained with immunohistochemistry (IHC) and hematoxylin and eosin (HE). The expression of CD-68 and CD-163 macrophages was identified with a monoclonal antibody immune-peroxidase (mAB) kit and a secondary anti-peroxidase antibody. The results from the antibody incubation were stained with a diamino-benzidine substrate (Bioss, USA) and were labeled using streptavidin-biotin horseradish peroxidase (LSAB-HRP) (Starr Trek, USA).
Statistical analysis
Statistical analysis was performed by using the SPSS software version 23.0 according to one-way ANOVA followed by Duncan Multiple Range Test (DMRT) when P<0.05. In addition, the parametric data were presented as mean ± SD.
RESULTS AND DISCUSSION
Blood urea nitrogen and serum creatinine concentration
The concentration of blood urea nitrogen (BUN) in the control group (P0) and treatment group (P2) were 23.62 mg/dL and 26.61 mg/dL, respectively (Table 1), which is in the normal value. In the positive control group (P1), the BUN level was 47.18 mg/dL. This value is significantly higher than those negative control and treatment groups using CA leaves extract (p<0.05). On the other hand, the serum creatinine (SC) level in the control group (P0) was 0.45 mg/dL, while it was 0.78 mg/dL and 0.50 mg/dL in the P1 and P2 groups, respectively. Serum creatinine and blood urea nitrogen can be used as indicators to evaluate kidney function. Under current investigation, we found that treatment with CA leaves extract (P2) significantly decreased the blood urea nitrogen (BUN) and serum creatinine (SC) compared with induced rats (P1).
This finding indicates that feeding the Coleus amoinicus extract can restore blood biochemistry to normal value in the treatment group induced by uric acid. According to previous studies, the induction of uric acid caused acute kidney failure indicating the increase of BUN and serum creatinine levels. Further negative effects of uric acid accumulation in the kidneys are increasing the incidences of hypovolemia, dehydration, and acute inflammation in the cells (Giknis and Clifford, 2008). The accumulating effect can also promote necrotically and inflammation in the renal tubules (Kensara, 2013; Shimada et al., 2009).
TGF-β1 and glutathione peroxidase concentration
For the TGF-β1 concentration, Table 1 showed that induced rats receiving CA leaves extract (P2) had significantly higher TGF-β1 concentration in comparison to other groups (p<0.05). In the negative control, the TGF-β1 concentration was 50.39 pg/mL while in P1 and P2 the levels increased to 124.97 pg/mL and 189.71 pg/mL, respectively. The Glutathione peroxidase (GPx) concentration showed a similar trend to the TGF-β1 whereas the P2 was the significantly higher negative control group (0.51 mU/mL vs 0.25 mU/mL, Table 1).
The results suggest that CA extract can promote the TGF-β1 and GPx concentrations. TGF-β1 is an anti-inflammatory cytokine, autocrine or paracrine with the function to regulate cell proliferation, apoptosis, chemotaxis, immunity, inflammatory response, and tissue repair (fibrosis) of kidney tissue. TGF-β1 plays a pivotal role in the fibrosis process by stimulating extracellular matrix fibrogenic gene and protein mitogen kinase. Increasing TGF-β1 expression is an indicator of reduced renal cell damage (necrosis) due to uric acid induction and it indicates the repairing processes in renal tissue (Nishida and Hamaoka, 2008). The findings in the present study corroborate the previous study reporting that feeding Coleus amboinicus extract was effectively repairing the renal tissue damage on cisplatin-induced rats (Sahrial and Solfaine, 2019). The cytokine TGF-β1 can regulate the formation of the extracellular matrix, collagen genes, and the synthesis of the extracellular matrix of mesangial cells on diabetic nephropathy. Abundant production of extracellular matrix (ECM) leads to fibrosis, thickened glomerular basement membrane, and sclerosis in the tubules interstitial and glomerular regions (Xie et al., 2015). A previous study reported that the cytokine TGF-β1 could induce profibrogenic genes by activating the EGF and angiotensin-II and endothelin-1 receptors in the renal tissue fibrogenesis. In uric acid induction, it is known that tubular cell fibrosis is influenced by the cytokine TGF-β1 (Martin et al., 2009). Furthermore, the result of GPx analysis indicates that
Table 1: Comparison of blood urea nitrogen (BUN), serum creatinine (SC), transforming growth factor-β1(TGF-β1) and glutathione peroxidase(GPx) on uric acid-induced rats among treatments
| Group | BUN (mg/dl) | Serum creatinine (mg/dl) |
TGF-β1 (pg/ml) |
GPx (mU/mL) |
| Control (P0) (n=8) |
23.62 a ±1.48 |
0.45 a ±0.06 |
50.39a ±31.38 |
0.25a±0.11 |
| Uric-induced (P1) (n=8) |
47.18 b ±13.45 |
0.78 b ±0.06 |
124.97b ±28.99 |
0.42 b±0.26 |
| Uric+CA extract (P2) (n=8) |
26.61 c ±8.35 |
0.50 c ±0.08 |
189.71c ±58.62 |
0.51 c ±0.27 |
Superscript in the same column indicate a significant difference of p ≤0.05
treatment using Coleus amboinicus extract could increase the activity of GPx in rats. Antioxidant enzymes are the defense system against free radicals. Radicals such as H2O2 can be neutralized by peroxidase. GPx antioxidant enzyme activity is regulated by people’s behavior in food consumption including dietary supplement intake and smoking (Lee et al., 2006).
Histopathological analysis
The histopathological profile of kidney tissue for each experimental group was analyzed for glomerulus and tubulointerstitial staining with hematoxylin-eosin (H and E) and immunohistochemistry (IHC). The expression of macrophages was identified in the area of tubules and glomerular cells in kidney tissues (brown aggregate) of treatment groups (Figure 1). Identification of macrophage expression was performed using the ED-1 and ED-2 antibody which can recognize the CD-68 and CD-163 expression in phagosome cells from blood monocytes and tissue macrophages. The appearance of macrophages in the inflammatory process indicates the phagocytosis process.
The results of this study showed that the expression of CD-68 and CD-163 macrophages increased in the positive control group (P1) and the treatment group using CA plant extract (P2), while there was no positive immunoreaction of macrophage expression in P0 group. The expression of macrophages observed in this study suggested that inflammation occurred in the P1 and P2 groups due to uric acid induction. The phagocytic activity of macrophages in the inflammation area is mediated by pro-inflammatory compounds and cytokines monocyte chemoattractant protein-1 (MCP-1) acute injury of renal tissue (Nishida and Hamaoka, 2008; Juniantito et al., 2013). In the P2 group, however, the inflammatory condition was effectively compensated with CA plant extract treatment as shown by decreasing the BUN and serum creatinine concentrations in the blood plasma.
On the other hand, Figure 1 showed that the expression of ED-1 macrophage cells increased after treatment and it was widely distributed in the tubular area in uric acid-induced nephrotoxicity rats (P1). In addition, the expression of CD-163 macrophage cells was also found to be higher in mesangial cells in the P1 group. The ED-1 and ED-2 macrophage expressions in this group were followed by an increase in serum creatinine and BUN levels. It has been suggested that the expression and activity of macrophage (Mø) cells are derived from the differentiation of blood monocyte cells, tissue cell origin, and dendritic cells. The CD-163 macrophage expression can be recognized by the ED-2 antibody, a macrophage cell that functions in antigen phagocytosis, induces inflammatory mediator compounds, influences cell apoptosis, and induces growth factors during the neutralization of infection with microorganisms (Nelson et al., 2011; Nishida and Hamaoka, 2008).
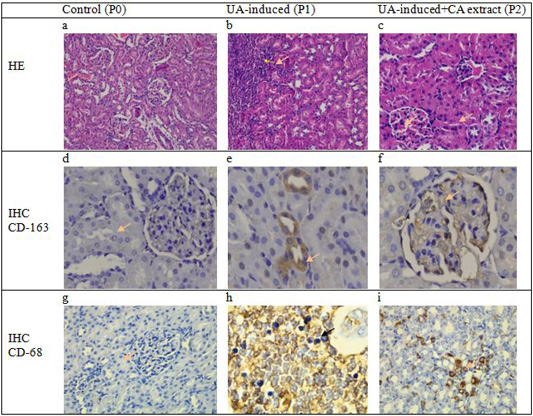
Figure 1: Cross-section of glomerulus and tubulointerstitial of rats showed no change in the structure of the epithelial and mesangium in the control group (a). However, there were severe infiltration cell, vacuolar degeneration, and necrotic epithelium in the area of glomerulus and interstitial tubules on induction group (b) meanwhile decrease the number of inflammation and alteration epithelial cells were observed in the histopathological cross-section of the renal tissue of rats in the group that received Coleus amboinicus. (c). No specific expression of macrophage CD-68 nor positive cells of macrophage CD-163 in the control group (a.d.) Expression of macrophage CD-163 positive cells is observed in interstitial and tubules (e) but decreased numbers of CD-163 expressions in the group with received CA extract (f). Meanwhile, the expression of macrophage CD-68 is also observed an increase in the area epithelial tubularcells in the treatment group (h) and decreased number of positive cells in the group with Coleus amboinicus extract (i) (40 x; IHC, counterstained hematoxylin).
CONCLUSIONS and Recommendations
In conclusion, the anti-inflammatory effect of Coleus amboinicus leaves extract was confirmed in this study as it could effectively reduce the blood urea nitrogen, serum creatinine, Glutathione peroxidase (GPx), and the TGF-β1 concentrations in uric acid-induced rats receiving the CA leave extract. The anti-inflammatory effect is associated with an increase of GPx concentration in blood that can inhibit acute kidney failure by restore BUN and serum creatinine to normal concentrations and inhibit renal tissue necrotic by increasing TGF-1β and decreasing macrophage CD-68/CD-163 expressions in uric acid-induced nephrotoxicity rats.
ACKNOWLEDGEMENTS
This research was funded through research grant No.32/LPPM/UWKS/V/2019, Kopertis VII, Ministry of Research, Technology and Higher Education, Republic of Indonesia.
NOVELTY STATEMENT
The role of Coleus amboinicus leaves extract as an anti-inflammatory by its mechanism in increasing antioxidant activity and restoring kidney function had not been done in previous studies. Feeding of Coleus amboinicus (CA) leaves extracts significantly restored serum biochemical parameters to normal control values on uric acid-induced nephroptoxicity rat.
AUTHOR’S CONTRIBUTION
This research designed and performed the study by RS. LM and RS collected the sample and analysis the data. The first draft of the manuscript was written by LM and S. AI, A, RS and LM reviewed the final draft and revised the manuscript. All authors have approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES





