Advances in Animal and Veterinary Sciences
Research Article
Peroral Administration of Sumbawa Horse Milk Could Inhibit Inflammation in The Duodenal Tissue of Inflammatory Bowel Disease (IBD) Animal Model Based on IL-6 cell Expressions and Inflammatory Cell Count
Nurina Titisari1*, Tiara Widyaputri2, Oktalita Kusumawati3, S. Prasetyawan4
1Department of Physiology, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; 2Department of Clinical Pathology, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; 3Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; 4Departement of Chemistry, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, Indonesia.
Abstract | This study aimed to determine the preventive effect of Sumbawa horse milk (SHM) on elevated IL-6 expression and the inflammatory cells in the duodenum of Inflammatory Bowel Disease (IBD) animal models. As an IBD animal model, 20 white male rats (Rattus norvegicus) Wistar strain were divided into five groups, i.e. T1 group (negative control), T2 group (positive control), and Sumbawa horse milk groups with different dose of administration that were T3 (0.5 mL/rat), T4(1 mL/rat), and T5 (1.5 mL/rat). The IL-6 expressions level was obtained by flow cytometry method from duodenal tissue, while the number of inflammatory cells was determined by duodenal histopathology examination. Data analysis in this study was quantitative data analysis which used statistical tests with One-Way ANOVA (α 95%) and continued with Tukey’s Test if significant difference was found. The results showed that an increase in IL 6 expression in all groups. The same thing was seen in the results of the inflammatory cell count, showed decrease in all groups. The conclusion in this research was SHM therapy at a dose of 1 mL (T4) and 1.5 mL (T5) could prevent the increase of IL-6 level and elevation of inflammatory cells in the duodenum of rats compared to the positive control group (T2).
Keywords | Lactoferrin, Lysozyme, Mare, Rats, Rattus norvegicus
Received | February 17, 2021; Accepted | May 24, 2021; Published | August 15, 2021
*Correspondence | Nurina Titisari, Department of Physiology, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; Email: nurina_titisari@ub.ac.id
Citation | Titisari N, Widyaputri T, Kusumawati O, Prasetyawan S (2021). Peroral administration of Sumbawa horse milk could inhibit inflammation in the duodenal tissue of inflammatory bowel disease (IBD) animal model based on IL-6 cell expressions and inflammatory cell count. Adv. Anim. Vet. Sci. 9(10): 1547-1552.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1547.1552
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Titisari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The cause of Inflammatory Bowel Disease (IBD) is still unknown, it is suspected that its etiopathogenesis is influenced by genetic factors, failure of immune regulation, exogenous factors, and the role of intestinal flora. In addition to these causes, IBD can be caused as a side effect of non-steroidal anti-inflammatory drugs (NSAID) such as indomethacin (Long et al., 2016). Even though indomethacin is often used in the treatment of gastrointestinal diseases, it also has a side effect i.e. reducing prostaglandin synthesis. Prostaglandin synthesis decrease will lead to a reduction of mucus production in the intestine and colon where it functions as a protection against viruses and pathogenic bacteria (Takeuchi et al., 2010). It can also increase the production of free radicals in the body, thus causing damage in the digestive organs, and is characterized by a reduced number of microflora (Brown et al., 2014).
IBD is indirectly caused by a reaction in hypersensitivity immune system unable to distinguish antigens, including digestive tract bacteria and feed Inflammatory Bowel Disease (IBD) occurrence preceded by the activation of mucosal T cells and macrophages that have increased expression then as a trigger for inflammation can stimulate the production of pro-inflammatory cytokines for example TNF α, IL-6, IL-18 and IL-1β (Guan, 2019). These pro-inflammatory cytokines can increase the production of excessive free radicals. Excessive free radicals can cause oxidative stress. Oxidative stress can be neutralized by antioxidant compounds. Thus, exogenous antioxidant compounds are needed to suppress free radicals that react with cell components and cause damage to organ cells (Tania, 2018).
Research has proven that horse milk which is consumed as human food can have several beneficial effects on health (Bareto et al., 2019). Sumbawa horse milk (SHM) can work as a probiotic. Probiotics can alter the flora of gastrointestinal tract by competitive mechanisms, produce antimicrobial substances, or influence local immune responses (Hemarajata and Versalovic, 2013). The study showed that Sumbawa horse milk has a high nutritional content and contains a bioactive protein component with a low molecular weight that shows the presence of lysozyme compounds and antithesis compounds and contains 11 types of amino acids (essential and non-essential) and 12 types of fatty acids (saturated and unsaturated) (Yuniati and Sahara, 2012). Lactoferrin has a strong bacteriostatic (anti-bacterial) activity due to its ability to bind metal ions which are vital to living things (Bures et al., 2011). According to Yuniati and Sahara (2012), lactoferrin in horse milk has the potential to reduce the inflammatory process and function as an antioxidant, while lysozyme acts as an anti-inflammatory agent by limiting the migration of neutrophils to damaged tissue.
From the above background, this study was conducted to determine the preventive effect of Sumbawa horse milk on the expression of interleukin-6 (IL-6) and the number of inflammatory cells in the duodenum of Inflammatory Bowel Disease (IBD) animal model.
MATERIALS AND METHODS
This study used a complete randomized design with 20 male rats (Rattus norvegicus) Wistar strain aged 8-12 weeks with an average weight of 200 grams. Animals were divided into five groups with 4 replication, i.e. negative control group (T1), positive control group (T2), and preventive groups that was T3 (0.5mL /rat), T4 (1mL/rat), and T5 (1.5mL/rat) induced with SHM for 21 days. Sumbawa horse milk in this research was obtained from Dompu Regency, Sumbawa Island, NTB (West Nusa Tenggara).
To create animal model, indomethacin was induced orally using a gastric intubation at a dose of 15mg/kg BW per rats (Aulanni’am et al., 2012) on all rats except in T1 group on day 15. The dilution of indomethacin was 45 mg in 4 mL of corn oil as the solvent (Bures et al., 2011). This research has been approved by Brawijaya University Research Ethics Commission (KEP-UB) Number 1046-KEP-UB.
Measurement of IL-6 level by flow cytometry
Duodenums obtained in this experiment were crushed with the syringe base in 5 mL PBS and then filtered with wire and put into 15mL propylene up to a certain volume. After that homogenates were centrifuged at 2500 rpm for 5 minutes at 10oC. The centrifugation supernatant was removed, leaving the pellets. The pellets were resuspended with 1mL PBS by piping. The results of the suspension were divided into several 1.5 mL microtubes containing 0.5 mL PBS as much as 50 µL for each microtube. The isolated cells were counted. The suspension was then re-centrifuged at 2500 rpm for 5 minutes at 10oC. The pellets from centrifugation were taken and then stained by adding 50 µL fixative solution, then incubated for 20 minutes at 4oC in a dark room. After that, 500 μL of Intracellular Staining Permeabilization Wash Buffer was added then homogenized and centrifuged again at a speed of 2500 rpm for 5 minutes at 10oC. After the pellet was obtained, a specific antibody (Rat antibody IL 6) was added to the cell as much as 50 µL and then incubated for 20 minutes at 4oC in a dark room. PBS 400 µL was added and the mixtures were then put into the cuvette flow cytometry to be smoothed.
Inflammatory cells count
The duodenal tissue was examined under a microscope at 400x magnification. The calculation of the number of inflammatory cells was carried out in 5 fields of view, then the average calculation was performed. The inflammatory cells counted is the total number of all leukocyte differentiations that was found, which is neutrophils, eosinophils, lymphocytes, and macrophages (Titisari et al., 2020).
Data analysis
The number of IL-6 expressions earned from the flow cytometry method and the number of inflammatory cells was analyzed quantitatively by One Way analysis of variance (ANOVA) test. Tukey’s test was performed if there was a significant difference (α 95%).
RESULT AND DISCUSSION
Preventive effect of Sumbawa horse milk on IL-6 expression in animal model duodenum
Normality and homogeneity test showed that the data are normally distributed and homogeneous, so that this data can be tested with one-way ANOVA test. ANOVA test showed there were significant differences within groups (p<0.05). The Tukey test results revealed different notations shown in Table 1 and found that the results of IL-6 production in the negative control group were significantly different with the positive control group. This means indomethacin induction for Inflammatory Bowel Disease (IBD) was proven to have a significant impact on increasing IL-6 levels in rat.
Table 1: Total expression of IL-6 in rat duodenum showed Sumbawa horse milk (SHM) groups (T4 and T5) were significantly different with positive group (T2) but not significantly different with negative group (T1) (P<0.05).
| Groups | Average expression of IL-6 ± SD (% cells) |
| T1 (Negative control) |
13,00 ± 2,17a |
| T2 (Positive control) |
26,90 ± 2,86c |
| T3 (0.5 mL SHM) |
21,45 ± 0,69bc |
| T4 (1 mL SHM) |
19,06 ± 3,18ab |
| T5 (1.5 mL SHM) |
15,42 ± 4,74ab |
Note: a, b dan c notation showed that there is a significant difference (p<0,05).
Interleukin 6 (IL-6) is normally produced in the body as a product of the body’s normal metabolism to maintain homeostatic conditions of the immune system (Vielhaeur et al, 2005). The negative control group (T1) showed the lowest average IL-6 expression level (13,00 ± 2,17a % cells) within groups (Table 1). On the contrary, the positive control group (T2) showed the highest expression level (26,90 ± 2,86 % cell). An increase in IL-6 indicates inflammation in the animal model due to indomethacin induction. Indomethacin inhibits the formation of COX-1 and COX-2, but more effectively inhibits the role of COX-1 prostaglandin formation. Decreased prostaglandin causes a decline of protection by the duodenum mucosal barrier that it can trigger inflammation (Wallace, 2008). In addition, indomethacin can also trigger the formation of ROS (Reactive Oxygen Species). Excessive ROS will stimulate Nuclear Factor activation Kappa B (NF-kB) which is a transcription factor in regulating the expression of pro-inflammatory cytokine cells such as IL-6 (Brasier, 2010).
In the SHM group, there was a lower IL-6 expression compared to positive control group. T4 group (1 mL SHM) and also T5 group (1.5 mL SHM) showed significant difference with T2 group but no difference with T1 group. T3 group showed the opposite result. This means that at a dose of 1 mL and 1.5 mL of Sumbawa horse milk can prevent an increase in IL-6 levels compared to positive controls. The prevention of increased IL-6 expression was probably due to lysozyme and lactoferrin bioactive contents contained in Sumbawa horse milk.
Lysozymes and lactoferrin work synergistically as antimicrobial and anti-inflammatory (Uniacke-Lowe et al., 2010). According to Hermawati (2005) and Reni et al. (2013) SHM contains lactoferrin components which can inhibit the respiratory burst process. The inhibition in the respiratory burst process can inhibit releasing free radical products that can destroy cell membrane and cell insides, such as fat, protein, and amino acid. And also, cytokine pro inflammatory. In addition, the ability of lysozyme to limit the migration of neutrophils to damaged tissue provides the possibility to use lysozyme as an anti-inflammatory agent (Yuniati and Sahara, 2012). Horse milk influences the modulation of the inflammatory process through its effect on decreasing inflammation cell chemotaxis processes, allowing it to treat recurrent inflammation (Ellinger et al., 2002).
In addition, Sumbawa horse milk in this research has a high content of lactic acid bacteria which is around 6.7x106 cfu/ mL. Lactic acid bacteria in Sumbawa horse milk (Sujana et al., 2008) can improve the digestibility of lactose (Zubillaga et al., 2001), increase the growth of intestinal microflora, stimulate the immune system and inactivate toxic compounds (Astawan et al., 2011; Kaur et al., 2002). According to Diding et al. (2008), SHM has probiotics that can improve the function of the intestinal mucosal immunologic barrier through pathogenic microbial excretion, restore normal intestinal mucosal permeability in allergic or inflammatory diseases, and decrease pro-inflammatory cytokine products.
Preventive effect of Sumbawa horse milk on inflammatory cells in animal model duodenum
The microscopic observations in duodenum tissue showed shortening of duodenal villi, epithelial erosion and the presence of inflammatory cell infiltration in the T2 group (Figure 1). In some parts of lamina propia villi an aggregation of erythrocyte cells was also found, which indicated vasodilation of blood vessels as a sign of inflammation. On microscopic histopathology observations, duodenal tissue in the SHM group (T3, T4 and T5) (Figure 1) showed an improvement of duodenal villi erosion and appeared to have normal size and length.
To count the inflammatory cell, we used microscope with 400x magnification (Figure 2). Based on statistics, normality and homogeneity test showed that data is
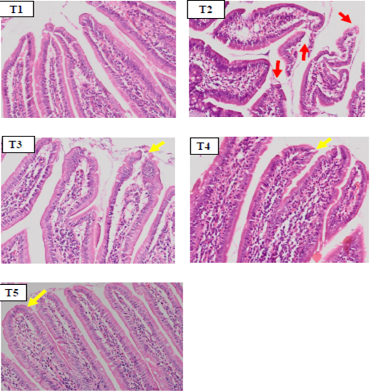
Figure 1: Hispathology of rat duodenum (Rattus norvegicus) with HE staining 100x magnification showed there was an erosion on duodenal villi epithelial (red arrow) in positive control group (T2) due to indometasin. The SHM group (T3,T4,T5) showed a improvement in duodenal villi epithelial damage.
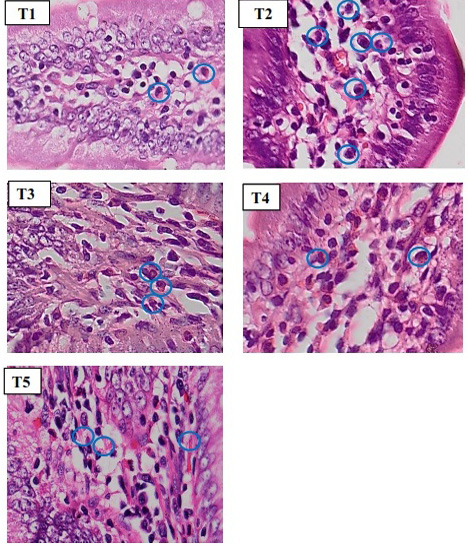
Figure 2: Histopathology of rat duodenum (Ratus morvegicus) (HE staining 400x magnification) revealed that group T2 contained more inflammatory cells (blue circle) than in all groups.
normally distributed and homogeneous. One-way ANOVA test showed a significant difference within groups (p <0.05). Tukey test was performed and the results are displayed in Table 2.
Table 2: Average number of inflammatory cells of rat duodenum (Rattus norvegicus) in the Sumbawa horse milk (SHM) groups (T4 and T5) were significantly different with positive group (T2) (P<0.05) but not significantly different with negative group (T1) (P>0.05).
| Groups | Average inflammatory cell counts ± SD (% cells) |
| T1 (Negative control) |
7,95 ± 2,19a |
| T2 (Positive control) |
19,7 ± 2,62c |
| T3 (0.5 mL SHM) |
14,85 ± 2,14bc |
| T4 (1 mL SHM) |
12,7 ± 2,94ab |
| T5 (1.5 mL SHM) |
9,52 ± 2,13a |
Note: a, b dan c notation showed that there is a significant difference (p<0,05).
In the microscopic observations of duodenal tissue, the negative control group (T1) showed the lowest number of inflammatory cells (7,95±2,19a cell/view) within the groups. Duodenal villi are long, normal and have no signs of inflammation. Lymphocyte cells appeared because lymphocytes are natural defense in digestive organs originating from the GALT (Gut Associated Lymphoid Tissue) system. Neutrophils will only be in circulation for 7-10 hours, before finally migrating to the network and living several days in the network (Baratawidjaja and Rengganis, 2014).
The microscopic observations of duodenal tissue histopathology in negative control group (T1) showed a normal duodenal image, such as long duodenal villi, epithelium cell was simple columnar, and the presence of goblet cells. Whereas positive control group (T2) showed shortening of duodenal villi, epithelial erosion and inflammatory cell infiltration. In some parts of the lamina propia, there was an aggregate of erythrocyte cells which indicate vasodilation of blood vessels as a sign of inflammation. The result of inflammatory cells counts revealed that T2 group has the highest number (19.7± 2.62c cells / view) in all group. The T3 group (14.85±2.14bc cells/ field of view), T4 (12.7±2.94ab cells / field of view), and T5 (9.52±2.13a cells/ field of view) showed that SHM groups were able to prevent the increase of inflammatory cells when compared to the T2 group. In addition, all SHM groups on the histophatological review showed a minor damage in the duodenal villi and an increasing number of goblet cells when compared with positive controls (T2).
The results of inflammation cell observation are in line with the results of IL 6 expression obtained previously. IL-6 acts as a proinflammatory mediator that will mobilize inflammatory cells in the inflammatory area (Scheller et al., 2011). This results in the manifestations of inflammation and inflammatory cell infiltration that can be seen in the histopathology sample. Inflammation by inflammatory cells occurs due to the activation of pro-inflammatory cytokines such as IL-6, thus causing the initiation of inflammatory cell infiltration at the site of inflammation. Inflammatory cells that dominate the histopathological areas of duodenal damage are macrophages, lymphocytes and neutrophils (Jaeger et al, 2012). These cells indicate that inflammation in Inflammatory Colon Disease (IBD) due to indomethacin induction is a chronic inflammation.
Sumbawa horse milk has bioactive content, namely lysozyme and lactoferrin. Lactoferrin has an important role in binding Fe in the intestinal mucosa and acts as a bacteriostatic agent. Its presence in neutrophils and their release during inflammation reinforce the notion that lactoferrin also plays a role in phagocytes destruction and immunity. Lysozyme is a protein compound that contains antibiotics that can destroy some bacteria. The ability of lysozyme to limit the migration of neutrophils to damaged tissue gives the possibility to use lysozyme as an anti-inflammatory (anti-inflammatory) agent (Yuniati and Sahara, 2012). These compounds will suppress the activation and migration of neutrophils and other inflammatory cells into the duodenal tissue so that the inflammatory reaction that occurs in the duodenal tissue and the amount of inflammatory cell infiltration could be prevented.
The bioactive compounds in horse milk are specific parts or fragments of protein that are reported to have several antioxidant mechanisms, including: As a scavenging radical, mineral chelating, metal reducing agent, and protector. Where antioxidants have an important role to protect the body from conditions of oxidative stress. Elias et al. (2008) stated that the amino acid histidine has an imidazole group which can function as a hydrogen giver and a free radical hydroxyl fat scavenger. This is largely determined by the specificity of the protease enzyme used so that it can work synergistically or antagonistically with other antioxidants (Phelan et al., 2009).
Conclusions and Recommendations
Based on the results of this study, it can be concluded that the administration of Sumbawa horse milk per oral in an animal model of Inflammatory Bowel Disease (IBD) would result in a lower amount of IL 6 expression and decreased the number of the inflammatory cells in the duodenal organs compared to the positive control group (T2). The optimum dose in this research was at a dose of was 1.5 mL (T5 group).
ACKNOWLEDGEMENTS
The present scientific research was financially funded by Faculty of Veterinary Medicine Research Fund, 2019, Brawijaya University, Malang, Indonesia.
Novelty Statement
Our novelty exploring the possibility of Sumbawa Horse Milk (SHM) as an alternative natural product to prevent inflammatory bowel disease (IBD).
AUTHOR’S CONTRIBUTION
The authors have declared no conflict of interest.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES





