Advances in Animal and Veterinary Sciences
Research Article
Effect Provision of Addition Urea Molasses Multi-nutrient Moringa Block (UM3B) feed to Bali Cows on Follicular Dynamics Profile During Estrus Synchronization
Abdul Malik1*, Siti Erlina1, Aam Gunawan1, Neni Widaningsih1, Mawardi2, Rizkie Elvania1
1Department of Animal Science. Faculty of Agriculture. Islamic University of Kalimantan M A B Banjarmasin. South Kalimantan -Indonesia; 2Animal Husbandry Agency, Tanah Laut District, Province of South Kalimantan-Indonesia.
Abstract | The objective to investigate the effects of the addition of UM3B on follicular dynamics profile after the second injection of PGF2α. Methods: In this experiment. A total of 20 Bali cows were used for this research. The average weights of the cow were 325-350±9.98 kg, cycling of estrus normally, and aged 3-5 years. All cows were divided into two treatment groups. The first group (n =10) was supplemented by UM3B dose of 350 g/head/day, and second group (n =10) was not supplemented with UM3B. The parameters were evaluated include of locations and sizes of the follicles, corpus luteum (CL) regression, and dominant follicles were assessed by a Ultrasonography (USG scan). Profile of estrus response after second injection PGF2α was observed manually. Results: Diameter of follicle ovulation, speed of follicle growth, length of the follicle growth phase, and follicular wave were significantly different (P<0.05) among group 1 and group 2. Whereas, the diameter of the follicle during injection, ovulation time, and estrus cycle length was not significantly different (P>0.05) among group 1 and group 2. While, the diameter of the corpus luteum during injection, diameter of corpus luteum during estrus, and speed of corpus luteum regression were not significantly different (P>0.05) between group 1 (with UM3B) and group 2 (without UM3B). Conclusions: The addition of UM3B could be improved by the diameter of follicle ovulation, speed of follicle growth, length of the follicle growth phase, and follicular wave in Bali cows.
Keywords | UM3B, Bali cow, Follicular dynamic, Estrus synchronization.
Received | March 14, 2021; Accepted | May 12, 2021; Published | July 28, 2021
*Correspondence | Abdul Malik, Department of Animal Science. Faculty of Agriculture. Islamic University of Kalimantan M A B Banjarmasin. South Kalimantan -Indonesia; Email: sidol_99@yahoo.com
Citation | Malik A, Erlina S, Gunawan A, Widaningsih N, Mawardi, Elvania R (2021). Effect provision of addition urea molasses multi-nutrient moringa block (um3b) feed to bali cows on follicular dynamics profile during estrus synchronization Adv. Anim. Vet. Sci. 9(9): 1483-1488.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1483.1488
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Malik et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
One strategy to raise productivity and reproduction in Bali cattle is the synchronization of estrus. Estrus synchronization which is continued by Artificial insemination (IA) has become a regular procedure to improve the population of cattle. Furthermore, estrus synchronization is a good tool to facilitate a timely artificial insemination program (Kasimanickam et al., 2009). There are several kinds of procedures that can be used to synchronize estrus in cattle, like usage of Prostaglandin F2α or PGF2α (Holm et al., 2008; de Araujo Berber et al., 2002). The use of PGF2 alpha or its analogues to lyse the corpus luteum has become the most popular method for the estrus synchronization program (Jainudeen et al., 2000). There are several methods for using PGF2 alpha for estrus synchronization program in cattle, one of these programs is the 2-shot prostaglandin protocol. Prostaglandin or its analogues can cause regression of the corpus luteum (CL) in cows from day 5 until day 15 of the estrus cycle (Holm et al., 2008).
The main factor that influences the success of the estrus synchronization program followed by IA is the quality and number of feeds consumed. While Miah et al. (2000) revealed that the administration of urea molasses multi-nutrient block (UMMB) in crossbreed cattle could increase milk production and shorting calving intervals. A good feed is a balanced feed between nutritional needs with the feed provided. Urea molasses multi-nutrient moringa block (UM3B) is a complement of nutrition in cattle. UM3B, which contains of a lot of molasses, urea, minerals, and high moringa. It is a respectable additional feed to meet feed deficiencies in cattle. The moringa is a species of plant, comprising complete nutrients, which possesses anti-oxidant contents, protein, fat, magnesium, calcium, selenium, potassium, vitamin C, vitamin E, and polyphenol of compounds (Ayodele et al., 2014), which can play a role in helping the growth of microbes in the rumen. Or it is increasing the population of microbial in the rumen may significantly improve the protein consumption (Malik et al., 2019). Furthermore, Lucy et al. (1992) revealed that the pattern of development of follicular was pretentious by the status of energy balance on the stage of lactating. Follicular dynamics were influenced by an energy balance, and by other factors such as the stage of lactation, level of production of milk, consumption of energy-rich nutrients such as calcium salts of long-chain fatty acids (Wolfenson et al., 1995).
The success of Estrus Synchronization can be seen by the development of follicles in the ovaries. Changes in ovarian follicles in cattle are characterized by the development of follicular waves and regression of follicle during the cycle of estrus (Ginther et al., 1989). Regular cows usually show one, two, three, or four follicular waves during estrous cycle (Savio et al., 1988; Taylor and Rajamahendran, 1991). These follicular wave patterns look both in post-pubertal such as two or three waves until ovulation and pre-pubertal by nonstop growth of follicles (Adams, 1994).
Most studies of estrus synchronization with prostaglandin F2a only report the ability to produce estrus and its conception results after artificial insemination. Only a limited study has reported the development of ovulation follicles. The objective of the study was to evaluate effect of addition urea molasses multi-nutrient moringa block (UM3B) on follicular dynamic profile during estrus synchronization with PGF2α in Bali cows.
MATERIALS AND METHODS
Animals
The experiments were conducted in district Tanah Laut and teaching farm Bentok - Lab. Animal production Faculty of Agriculture-Islamic University of Kalimantan, Banjarmasin Province of West Kalimantan. A total of 20 Bali cows were used for the research. The average body weights were 325-350±9.98 kg and aged 3-5 years. The mean ambient temperature during the study period were 32-35 °C, and deworming and vaccination was routine every year. All the cows were 90-120d postpartum and were cycling of estrus normally. Their body condition score was evaluated on a rule of 1 to 5 (1=thin; 5=obese) by Ayres at al. (2009). The score of the cow’s body condition was subjectively given to describe overall body condition, fat cover, and flesh over the ribs, loin, and tail head. All cows were kept in the same pen and raised under a similar grazing system (various kinds local of grass) and added with a mixture of rice bran (byproduct of milling) at 1.5 kg/head/day (Malik et al., 2013). The approval of the animal ethics committee on the conduct of this study was number P01.12.19 from research institutions and community service- Islamic University of Kalimantan.
Experimental Design
All samples of Bali cows were divided into two treatment groups. The first group (n =10) was supplemented by UM3B dose of 350 g/head/day, and the second group (n =10) was no supplementation UM3B. Component configuration (%) of UM3B was adopted Malik et al. (2020). Estrus synchronization for all cows was injected by intramuscular PGF2α with a dose of 25 mg Dinoprost® (Glandins, Tad Pharmazeutisches Werk Gmbh, West Germany). The second PGF2 alpha injection was carried out 14 days later after the first injection with the same dose and manner. cows of estrus were observed visually to estrus like mounting, standing to be mounted, and number of mounts performed for a period of 2h. cows that are ready to be mount at least 3 times were considered to be estrus (Busch et al., 2008; Malik et al., 2011).
Ultrasound Examinations
The ovarian follicular growth was examined at 2-d intervals (n = 5 each group) through ultrasonography using an Aloka 500V ultrasound with a 7.5 MHz trans-rectal linear probe (Aloka, Wallingford, CT), trans-rectal linear transducer. Positions and sizes of the follicles and corpus luteum (CL) in the ovaries were drawn at each scanning, and the exact location of the follicles was recorded. Evaluate of dominant follicle was considered by the deviation of its growth rate when compared to the subordinate follicles of the similar wave, a dominant follicle was clear to be the biggest follicle (Sirois et al., 1988). The follicles wave was
Table 1: Characteristics of ovulation follicular in Bali cows
|
Treatments |
The maximum diameter of the follicle ovulation (mm) | Speed of follicle growth (mm/day) | Diameter of follicle during second injection of PGF2α (mm) | Length of growth phase (days) | Ovulation time (h) | Estrus cycle length (days) | Follicular wave (cycle) | SEM | P |
|
Without UM3B (n=5) |
10.18 | 1.02 | 5.79 | 5.20 | 90.13 | 21.01 | 2.08 | 0.04 | p≤0.05 |
|
With UM3B (n=5) |
13.24 | 1.97 | 5.01 | 7.40 | 89.56 | 20.80 |
4.82 |
Table 2: Size of corpus luteum in Bali cows with UM3B and without UM3B
|
Treatments |
Diameter corpus luteum during second injection of PGF2 α (mm) | Diameter corpus luteum at Estrus (mm) | Speed of corpus luteum regression (mm/day) | SEM | P |
|
Without UM3B (n=5) |
12.01 | 4.63 | 3.02 | 0.06 |
p≤0.05 |
|
With UM3B (n=5) |
12.90 | 4.01 |
2.92 |
Table 3: Average diameter follicle of ovulatory follicle after second injection of PGF2α
|
Treatments |
Day 14 (day of second PGF2α injection) | Day 15 after injection | Day 16 of estrus | Day 17 of estrus | SEM | P |
|
Without UM3B (n=5) |
3.38 | 7.92 | 10.58 | 12.90 | 0.03 | p≤0.05 |
|
With UM3B (n=5) |
3.81 | 11.38 | 14.16 | 15.08 |
identified, measured, and drawn in diagrams, while, the size of the corpus luteum was measured by the average of the longest and shortest diameters (Figure 1). Furthermore, the time of ovulating was determined by the disappearance of the dominant follicle with a suddenly when the last diameter follicle (DF) was observed on the ultrasonography monitor (Azizah et al., 2013).
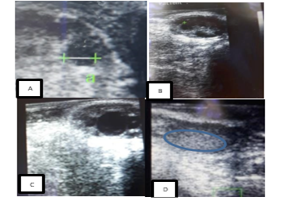
Figure 1: Ultrasound images of a cow’s ovaries in this study. (A) This image shows to get diameter of follicle or corpus luteum, (B) these images show five follicles, (C) these images show one dominant follicle, and (D) these images show of corpus luteum.
Data Analysis
The analysis of statistical was done using software SAS (Statistical Analysis System, version 9.2), through command Proc GLM. The data on follicular dynamics including number follicles, size of follicles, dominant follicles and performing of corpus luteum were assessed by analysis of variance, adopting α = 0.05 in a completely randomized design.
RESULTS
Profile of ovulation follicular
The characteristics of the ovulation follicle of the cow in groups 1 (with UM3B) and group 2 (without UM3B) were shown in Table 1. The diameter of the maximum of the follicle ovulation was significantly different (P<0.05) between group with UM3B and group without UM3B. Furthermore, the speed of follicle growth and length of follicle growth phase were significantly different (P<0.05) between group with UM3B and group without UM3B. The follicular wave was significantly different (P<0.05) among group 1 and group 2 (Table 1).
Diameter follicle and corpus luteum
Whereas, the diameter of follicle during injection of PGF2α, ovulation time, and estrus cycle length was not significantly different (P>0.05) among group 1 and group 2. On the other hand, the diameter of corpus luteum during injection of PGF2α, the diameter of corpus luteum during estrus, and speed of corpus luteum regression were not significantly different (P>0.05) between group 1 (with UM3B) and group 2 (without UM3B) see Table 2.
Diameter follicles and profile of estrus after second injection
Furthermore, the diameter of ovulatory follicles after the second injection of PGF2α including days 15, 16, and 17, respectively were significantly different (P<0.05) between group 1 and group 2 (Table 3). Whereas, the profile of estrus response after the second injection of PGF2α was not significantly different (P>0.05) among group 1 and group 2 see Figure 2.
DISCUSSIONS
The pattern of follicular growth was affected by the status of energy balance in cows. While, follicular dynamics were influenced by reasons affecting energy balance, such as the stage of lactation, level of production of milk, consumption of energy-rich nutrients such as calcium salts of long-chain fatty acids and a lot of minerals others. Supplementation with UM3B in feed was significantly (P<0.05) to increase the diameter of the follicle, follicular wave, and Length of growth phase as compared to control group 2 (without UM3B). In cattle, feeding is one of the main reasons of anestrus and other reproductive disorders (Kumar et al., 2014; Pradhan and Nakagoshi, 2008). Thus finding was strengthen by D’occhio et al. (1990) who revealed that the bad effect of low body condition score (BCS) on ovarian cycle and pregnancy of rates in cows. Furthermore, Rasby et al. (1992) stated that nutrition restriction has a negative influence on LH release, to finally effect of the estrus cycle.
Supplementation of UM3B during less availability of nutrition could improve the body condition score and reproductive efficiency of anestrus on Bali cattle. The high content of urea, molasses, and moringa in UM3B can have a good impact in overcoming energy balance in Bali cows, so that it has an impact on increasing follicular dynamics including diameter follicle, follicular growth and the appearance of follicular waves compared to the control group without given UM3B (Table 1). The result of this research was strengthening by Llewelyn et al. (2007) and Zulu et al. (2002) who exposed that many researchers have claimed that energy shortage causes the incidence of functional disorders of the reproductive system, such as long-term ovarian dysfunction or a postponement in the onset of normal ovarian activity. Furthermore, Diskin et al. (2003) reported that energy shortage in cows was significantly affect development of the diameter of the dominant follicle. On the other hand, a decrease in feed intake may donate to negative energy balance which may lead to an abnormality and irregular follicle growth (McDougall et al., 2007).
The other parameters in this study were evaluated of the diameter corpus luteum and size of corpus luteum during estrus synchronization with PGF2α. Based on the results of this study about corpus luteum (CL) diameter during injection, Cl diameter during estrus, and speed regression of CL was no significant difference between cattle with UM3B and without UM3B. Whereas, the profile of the percentage response of estrus after the second injection of PGF2α was showed similar between group 1 and group 2 (Figure 1).
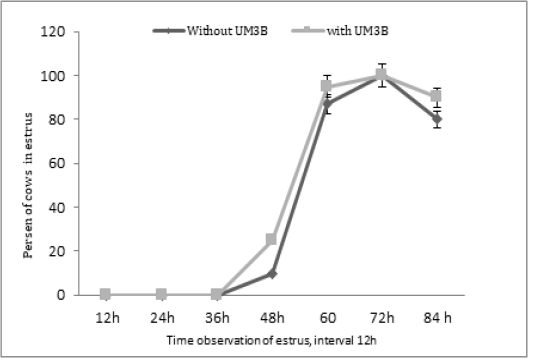
Figure 2: Percentage of estrus response after second injection of PGF2α in cows observed at 12h intervals (with UM3B or without UM3B).
The results of this research were strengthened by Malik et al. (2013); Cartmill et al. (2001); Pancarci et al. (2002) who stated that the higher percentage response of estrus among cows after the second PGF2α injection proposes that all of the cattle were in the same phase of luteal on the cycle. Furthermore, Smith et al. (2005) also revealed that PGF2 alpha was real only on luteal phase in corpus luteum.
CONFLICT OF INTEREST
All authors were declaration no conflict of interest
CONCLUSION
Base on the result of this research was decided that adding of UM3B on Bali cows could be improve of the diameter of follicle ovulation, speed of follicle growth, length of follicle growth phase, and follicular wave in Bali cows.
ACKNOWLEDGMENTS
The project of study was financed through Ministry of Research, and Technology - Republic of Indonesia, with number of contract 441/LL11/KM/2020.
AUTHORS’ CONTRIBUTION
A.Malik was coordinator of the research and analyzed and interpreted the data. Siti Erlina, Aam gunwan, and Neni Widaningsih in the study were supervisor of data collection and wrote draft manuscripts, Riskie and Mawardi was assistants of the collection of data.
REFERENCES





